Introduction
Numerous museums and art galleries are exhibiting a huge number of paintings and artworks. The paintings depict our life, our thoughts, feelings, and desires through a variety of subjects, objects, and colors. The paintings differ in their content, as well as in painting style or technique.
Technique in painting refers to the features of applying paint on canvas and the associated visual and optical effects. Techniques can be both traditional and original, or even whole complexes of techniques and methods. Knowledge of painting techniques allows an artist to create long-lasting works and make the best use of painting materials of available quality.
Speaking on painting technique, the composition of paints plays important role here, especially the properties of binders, that hold paint pigment particles together and get them fixed to base or ground surface, forming a paint layer. Strength of the paint layer, rate of its aging and destruction, as well as visual effects (pastel velvety, lacquer transparency, gloss, texture) depend on the binder.
Besides aesthetic perception, paintings are also of interest from a financial point of view, mainly as a capital investment option. To secure themselves against financial losses, collectors of the new formation turn to museums and expert organizations for value confirmation for paintings to be bought [1]. However, practice shows that art criticism, if relies upon traditional methods of paintings attribution only, as a rule, does not often provide a confident way to distinguish a fake from a genuine work. Only a detailed technological (forensic) examination, including a comprehensive analysis of paintings using a group of analytical methods, such as electron microscopy, IR spectroscopy, X-ray fluorescence analysis, etc., carried out by specialists with deep scientific background in laboratory research of paintings and knowledge of art materials, is really able to offer an answer on the time of creation of a particular work. Possible errors in determining the age of paintings are usually explained, on the one hand, by insufficient knowledge of artistic materials characteristic of different periods of time, and, on the other, by studies that do not fully disclose their specific features.
The latter problem can be successfully solved by using gas chromatography-mass spectrometry for detailed analysis of marker substances in composition of paintings. This method allows to determine many painting materials, features of binding paints, natural and technology related impurities, as well as to assess the degree of polymerization of the binding material.
The most common painting technique for several centuries has been oil painting, so it uses vegetable oils as main binder, that dries in the air and turns into a solid substance. Such oils are extracted from the seeds of various plants (flax, poppy, hazel, etc.). The most common method of obtaining oils is the method of cold pressing of seeds.
In the second half of the twentieth century, due to development of domestic paint industry in USSR along with synthesis of new materials, the composition of paints for painting has undergone significant changes. Paints based on the pentaerythritol esters of sunflower oil fatty acids, called “penta-oil paints” (1963), as well as on the basis of dehydrated castor oil renol (DCO) (1980), began to appear on the market. Despite the fact that castor oil belongs to the group of non-drying vegetable oils, subjected to dehydration (dehydration), it acquires the ability to dry, which also allows it to be used as a binder of paints. Thus, oil paints can be divided into three main types:
- paints based on drying vegetable oils,
- paints based on pentaerythrite esters of fatty acids (penta-oil),
- paints based on dehydrated castor oil (DCO).
In the course of this study, we evaluated use of gas chromatography-mass spectrometry (GCMS) method to determine types of binder in oil paintings along with possibility of detecting complementary chemical markers for dating paintings, as well as determining their authenticity.
Composition and properties of vegetable oils
Vegetable oils mainly consist of triglycerides (93-96. 5%), which are esters of the triatomic alcohol glycerol (in the form of a structural skeleton) and higher fatty acids (Fig. 1). Composition of acids in oil is variable and completely determines its properties and diversity, while glycerol for all oils is an invariable component.
The fatty acids of oils are divided into liquid unsaturated (unsaturated) – linoleic, linolenic, oleic and others, and into solid limit (saturated) – palmitic, myristic, stearic and other acids. As a percentage, the oils contain approximately 85-90% unsaturated acids and 7-10% marginal acids. The structural formulas of some common fatty acids in oil binder of paints are shown in Figure 2.
 Fig. 1. Block diagram of the structure of triglyceride. R1, R2, and R3 can form complexes with fatty acids
Fig. 1. Block diagram of the structure of triglyceride. R1, R2, and R3 can form complexes with fatty acids
Fig. 2. Common fatty acids of vegetable oils used as paint bindings.
| Palmitic acid (C16: 0) | |
| |
Stearic acid (C18: 0) |
| |
Oleic acid (C18:1) |
| |
Linoleic acid (From 18: 2) |
| |
α- Linolenic acid (C18:3) |
Differences in content of saturated and unsaturated fatty acids in structure of triglyceride are the main feature that distinguishes different vegetable oils, and, accordingly, binders of paints from each other [2]. Depending on what fatty acids and in what quantitative ratios are in oils, they are: drying, for example, linseed, hemp, nut, poppy; semi-drying, for example, cotton, maize, sesame, sunflower; non-drying, for example, castor, almond, olive and pistachio.
The quality of a particular oil used is entirely determined by the resistance of its binding effect, namely its oil film to various environmental influences. Strength of binding effect is characterized by the duration, nature and type of the drying process of the oil, and this process itself is extremely complex.
In the sun light and in presence of air, oil dries (oxidizes) from the surface and hardens. On the surface, a soft, and then gradually hardening oil film is formed, which holds the pigments and prevents the free access of oxygen, greatly slowing down the drying process of the underlying layers of oil. This process does not stop for 2-3 years, during which the picture first significantly adds weight, and then loses it again. Completely dry oil is a very brittle layer that easily cracks if bent slightly.
Linolenic acid contributes most to the drying rate of oils (to a lesser extent – linoleic acid). Therefore, linseed oil, which contains the most linolenic acid, dries faster than other oils. Nut oil, which contains a small amount of linolenic acid, but a lot of linoleic acid, dries slightly slower than linseed oil. Poppy oil, which consists of linoleic and oleic acids in the absence of linolenic acid, dries very slowly.
Study of oil paint binding fatty acid composition
Characterization of binding (or drying) oils used in painting has always been one of the main tasks in restoration and dating of paintings [3]. Currently, one of the most widely used approaches is based on determination of palmitic to stearic acid ratio index (C16/C18 or P/S) in binder composition by GCMS method [4-9]. This ratio does not depend on the aging process of the paints and is thus an ideal marker for oil identification [3]. This approach was first established by Mills in 1996 [10]. The main problem with using this approach is the large spread of index values published in literature (Table 1).
Table 1. The C16/C18 area ratio average values and standard deviations (in parentheses) measured in various oil binders, as per [3].
| Signal ratio index C16/C18 | Lit. reference | ||||
| Linseed oil | Nut oil | Poppy seed oil | Egg yolk | Egg/linseed oil | |
| 1,9 ± (0,5) | 3,3 ± (1,1) | 5,5 ± (2,5) | – | – | [10] |
| 1,2 ± (0,2) | 2,2 ± (0,3) | – | 2,4 ± (0,3) | – | [9] |
| 1,5 ± (0,1) | 2,5 ± (0,4) | 3,0 ± (0,5) | 1,9 ± (0,2) | – | [11] |
| 1,6 ± (0,1) | 3,3 ± (0,1) | 3,9 ± (0,1) | 2,5 ± (0,1) | 1,8 ± (0,4) | [7] |
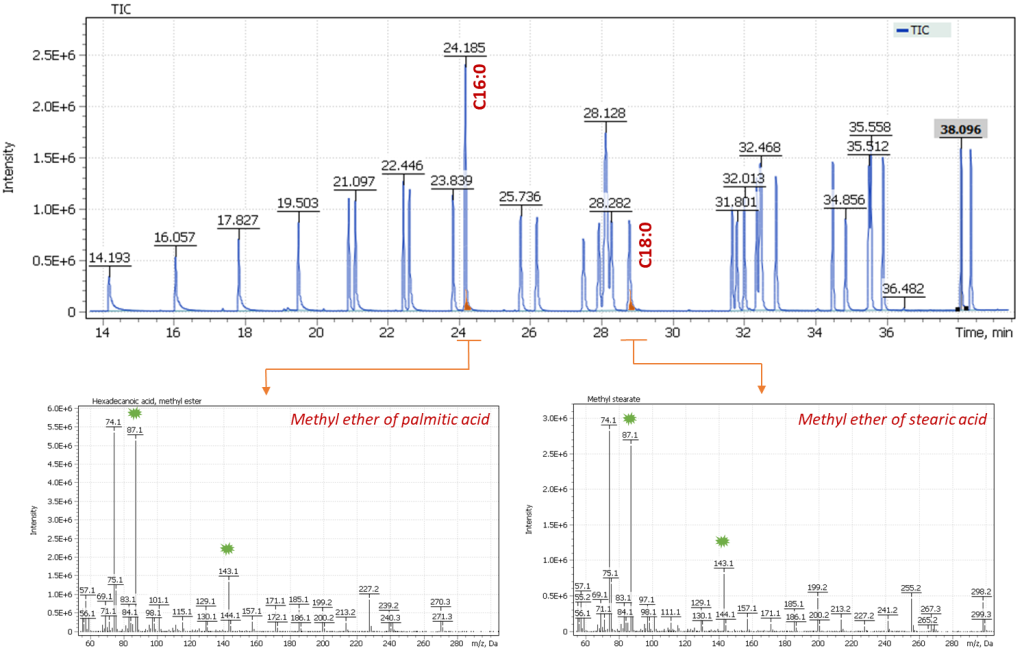
Fig. 3. Total Ion Current (TIC) Chromatogram of 37 fatty acid methyl esters, as well as extracted mass spectra (EI) of palmitic and stearic acid methyl esters.
The suitability of using the C16/C18 ratio is also justified by the constancy of the response factor-the peak integral: concentration for both fatty acids [3]. Notably, palmitic and stearic acids are rather closely eluting compounds on chromatogram and have a fairly similar structure, have similar characteristics of the ionization cross-section of the molecules and similar fragmentation paths in the ionization source. Such dependencies allow us to use selective signals of similar fragment ions (Approx. marked with green markers on the mass spectra below) to calculate the ratio of the presence of acids in the sample extract. Figure 3 shows a Total Ion Current (TIC) chromatogram of an analytical standard containing 37 fatty acid methyl esters, including signals of palmitic acid methyl esters (C16:0) and stearic acid (C18:0) acids, as well as their mass spectra.
The large variability in C16/C18 ratio index values found in literature has presumably to do with nonlinearity of analytical systems, when the response of the acid ratio became dependent on the dilution of the sample. Similar observations were demonstrated by a group of researchers led by Professor Tsakalof et al. and are described in detail in his work [12]. In the case described in the article, the nonlinear response of the device was associated with incomplete evaporation of the sample in the GC inlet. Use of a liner with deactivated glass wool improved sample evaporation and ensured linearity of the system response and independence of the P/S ratio from dilution factor of the sample [12].
Analysis of the acid ratio index table (Table 1) shows that the ratio differences are quite natural. In nut oil, the acid ratios are almost twice as high as in linseed oil, and the ratio index in poppy oil is also obviously higher (average index value 4) than in nut oil (average index value 2.8). The only non-obvious difference is the possibility of an error when identifying binders on pure linseed oil or a mixture of linseed oil with animal fats (egg/linseed oil). According to the literature data, in binders containing animal fats, a signal of polycyclic alcohol – cholesterol should also be present in the chromatogram profile, which makes this type of binder also easy to identify and separate [13].
During paint aging process, the loss of unsaturated monocarboxylic acids occurs with the simultaneous formation of dicarboxylic acids, especially azelaic acid, which can also be analyzed by chromatography-mass spectrometry [10]. In particular, high signals of dicarboxylic acids (mainly suberic, azelaic and sebacic) indicate the presence of aged oils in the picture. This effect is due to the fact that dicarboxylic acids, of which azelaic acid is the most common, are formed during self-oxidation of polyunsaturated acyl chain present in the binding oils [14]. Estimation of the ratio of azelaic acid to palmitic acid (A/P index) makes it possible to distinguish between different sources of triglycerides (vegetable or animal origin), since the amount of dicarboxylic acid formed during aging of animal fats is significantly less than in vegetable oils [14]. Values of the A/P index > 1 indicate presence of vegetable oils in composition of binding paints, while values of the A/P index < 0.3 are typical for triglycerides of animal origin, for example, for egg yolk. Intermediate values of the A/P index (0.3 < A/P < 1) are observed for artificial tempera, where a mixture of drying oil and egg yolk was used as an emulsion.
Besides, the sum of dicarboxylic acids SUM (D) can be used as an index to determine type of oil binder. According to literature, if the total content of dicarboxylic acids is more than 30% relative to the total content of all other acids in the sample, the binder is most likely of plant origin. If the amount of dicarboxylic acids was less than 10%, then a conclusion is made about the absence of vegetable oil and the use of animal fats is assumed [14].
Notably, quantitation of dicarboxylic acids should be carried out as quantitative analysis, using GCMS calibrations with respective analytical standards. As per literary sources, azelaic acid is prone to non-linearity of the signal response, especially at high concentrations, which can lead to greater variability in the results of the A/P ratio [3]. It is also obvious that the quantitative values of the A/P and SUM(D) indices strongly depend on the level of oxidation, decomposition and polymerization of the oil binder, so these indicators are influenced by many factors, in particular preheating the oil base before use, the age of the paint, the storage conditions of paintings and the interaction of the oil binder with pigments [14].
The quantification of glycerol, in addition to fatty acids and dicarboxylic acids, is also a useful index, namely, the calculation of the ratio of palmitic acid to glycerol (P/G) and azelaic acid to glycerol (A/G) helps to interpret the chemical transformations that occur during curing and aging of the oil binder [15].
And finally, ratio of oleic acid to stearic acid (O/S) is another additional significant index used to assess the degree of aging of oil binder (oxidation of the binder), because the double bond of oleic acid is particularly sensitive to oxidation and, therefore, its content in old paintings will be at a low level. The O/S index ≈ 0.1–0.2 will characterize a high degree of oxidation of the binder of paints and thus suggest a possible age of the studied paintings [16].
In the second half of the twentieth century, in artistic oil paints manufacturing, natural drying oils are replaced with their synthetic analogues to be used as a binder: transesterified, including penta-oil, alkyd oils and dehydrated castor oil (DCO). Identification of these new binders by legacy physical and chemical methods proved to be almost impossible, especially in samples of paint layers with a multicomponent composition. The optimal method for identifying new synthetic oils was gas chromatography-mass spectrometry with preliminary acid methanolysis of the test material [17]. Taking into account the fact that these binders were produced only in the domestic paint and varnish industry, they can serve not only as temporary, but also as a geographical marker for the examination of paintings.
Analysis of binders based on penta esters of sunflower oil fatty acids
Sunflower oil, like all fatty oils, also consists of glycerides. By special treatment in autoclaves at a temperature of over 200 degrees and a pressure of 25 atmospheres in the presence of water, the process of splitting the glycerides of sunflower oil into glycerol and fatty acids takes place. Next, the esterification of sunflower oil fatty acids is performed by combining them with a tetra-atomic alcohol-pentaerythritol, that is, the replacement of glycerol with a more active substance pentaerythritol to form pentaesters of sunflower oil fatty acids.
Compared to conventional sunflower oil, pentaesters acquire a number of new properties that play an important role when used as a paint binder:
- The drying rate of the oil is almost doubled.
- The oil forms elastic films that are not inferior in strength to the linseed oil film.
- The oil with pigment gets a good pasty without the addition of wax or aluminum stearates.
- The film of this oil practically does not turn yellow.
Thus, the pentaesters of sunflower oil fatty acids are not only not inferior to the best samples of linseed oil, but even surpass them in the most important and valuable properties for painting [18]. The first zinc whitewash based on the penta esters of sunflower oil fatty acids was released in 1960, so the detection of pentaerythritol is a convenient marker for dating paintings.
For the first time, I. A. Grigorieva proposed the method of identification of pentaerythritol in the composition of the paint binder [27]. She noted that in the IR spectra of penta-oil paints samples, the absorption band in the region of 1100 cm-1, attributed in oils to the valence asymmetric vibrations of the C-O group of triglyceride esters, has a lower intensity compared to the vibrations of the C-O groups in the high-frequency regions and is recorded as a characteristic arm. In addition, in the IR spectra of penta-oil paints in the region of 1060-1018 cm-1, there are a number of low-intensity bands that can be attributed to the vibrations of the C-O-H groups. Despite the fact that such characteristic peaks are observed only in paintings created near the 1960s (i.e., in the early 1960s). after the penta-oil paints appeared on the domestic market), in order to unambiguously correlate the data of spectroscopic characteristics and the presence of sunflower oil fatty acid pentaesters in binder, it is necessary to run a number of experiments using gas chromatography-mass spectrometry method to accurately identify components of the binder. In addition, the IR spectroscopy method has another drawback: since composition of the paint layer is often multicomponent, the intense absorption bands of some pigments or inert fillers (for example, barite, kaolin, etc.) may overlap the region of interest in the IR spectrum (Fig.4). In particular, Figure 4 clearly shows how intense absorption of barium sulfate in the region of 1200-900 cm-1 overlaps the region of the IR spectrum where pentaerythrites absorption bands are recorded. Therefore, the GCMS method providing confident and reliable results regardless of composition of the paint layer, is the most accurate in identifying the binder under investigation [27].
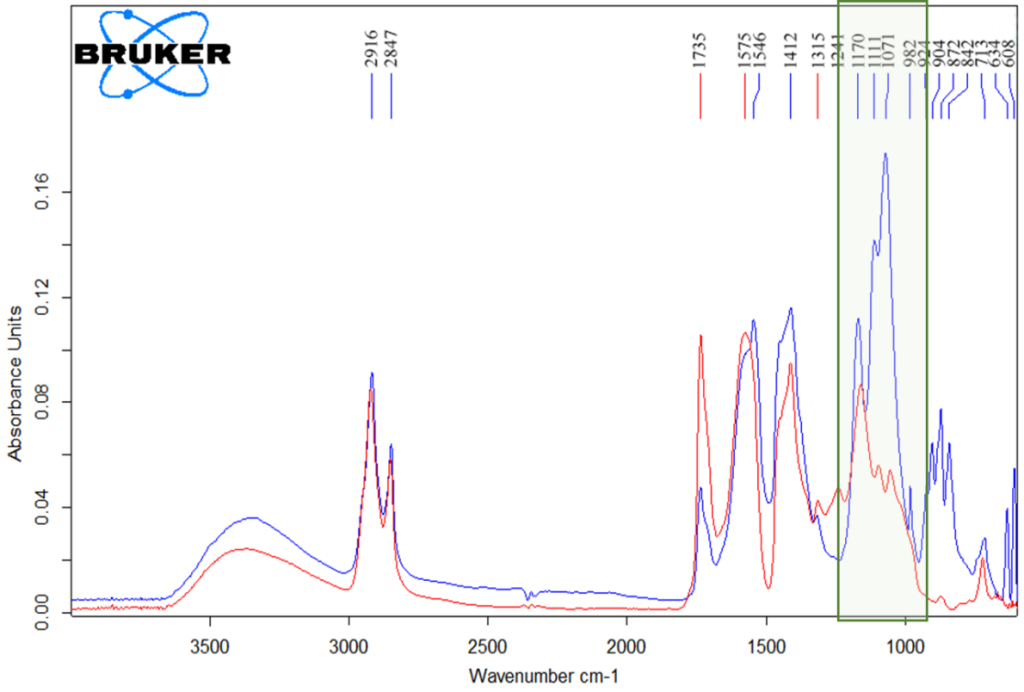 Fig. 4. IR spectra of penta-oil paint based on zinc whitewash (red color spectrum) and paint containing inert fillers and pigments (blue color spectrum).
Fig. 4. IR spectra of penta-oil paint based on zinc whitewash (red color spectrum) and paint containing inert fillers and pigments (blue color spectrum).
To detect presence of pentaerythritol in the composition of the binder tetra-atomic alcohol using a gas chromatography-mass spectrometric (GCMS) system, polyatomic alcohols (glycerin and pentaerythritol) are converted into full or partial trimethylsilyl derivatives during the sample preparation procedure, which makes it possible to reliably detect their presence in the organic fraction of the paint sample extract. The drawbacks of GCMS method is complexity of step-by-step sample preparation, as well as duration of chromatographic separation.
Analysis of binders based on dehydrated castor oil (DCO)
In the identification of dehydrated castor oil (DCO) by IR spectroscopy, similar problems are observed as in the study of penta-oil paints. To date, based on the IR spectrum, we can only assume DCO presense by the characteristic absorption in the region of 1600-1400 cm-1, the close intensity of the absorption bands 1165 and 1100 cm-1, as well as the low intensity of the absorption band 1240 cm-1 (recorded as a shoulder) [27] (Fig. 17). However, these areas are often overlaid with components that are part of the paint (for example, calcite / chalk or lead whitewash, characterized by intense absorption in this area). Therefore, the GCMS method is the most promising for study of this type of oil binder.
Dehydrated castor oil is obtained by heating castor oil at 180-200°C with the addition of various reagents. Raw materials are extracted from castor seeds by pressing or extraction with organic solvents. Castor dehydrated oil is a non-drying oil, while dehydrated oil can form solid films [19].
As noted earlier, in the USSR in the second half of the XX century, many art paints manufacturers began to use dehydrated castor oil to partially or completely replace linseed and other vegetable oils. Such a binder forms a sufficiently plastic film already at room temperature and, unlike other oils, such films do not turn yellow [19].
A large proportion of castor oil (80 %) is made up of glycerides of viscous ricinoleic acid, which contains only one unsaturated bond in a huge molecule. The rest is accounted for by glycerides of linoleic and oleic acids. When the OH group is dehydrated and removed by a dehydrating agent, such as sulfuric acid, a mixture of doubly unsaturated linoleic acids is formed, which are very similar in their properties to linseed oil.
Accordingly, it becomes quite difficult to detect the binder is not the original drying oil, but synthetic dehydrated castor oil. Fortunately, many reactions in organic chemistry do not go to the end, and some amount of ricinoleic acid in triglycerides is not dehydrated, and it is these trace amounts of ricinoleic acid that can be determined by gas chromatography-mass spectrometry in the form of a methyl ester of ricinoleic acid silylated by the OH group. Ricinoleic acid is identified only in castor oil and is not present in other oils.
Objects of study
Three paintings undergoing restoration, examination and research at the State Research Institute of Restoration (GOSNIIR, Moscow) were accepted for study (Fig. 5):
- The painting “The Reveller. Oleg Sataev”, artist V. Zubarev. Signed and dated 1972. The painting is painted on canvas using mixed media, 66. 0 cm. Collection of the Russian Abstract Art Foundation.
- The painting “Song of the Soul”, author unknown. Signature and date 1967 Oil on canvas, 86.0×116.0 cm. Private collection.
- The painting “Rebti. Portrait of a girl on the background of a waterfall”, artist S. N. Roerich. Signed and dated 1936. Mixed media on canvas (?), 61.5×92.0 cm. Collection of the State Museum of Oriental Art.
All works have signatures and/or dates of their writing. Previously, the soil and the paint layer of the paintings were studied by polarizing microscopy, IR spectroscopy and electron microscopy in the Research Laboratory at GOSNIIR, which made it possible to identify the pigment composition of the paint layers, as well as to make initial assumptions about the types of oil binders.
Fig. 5. Artworks used as objects for this study.
| “The Reveller. Oleg Sataev”, artist V. Zubarev. | Uknown artist, «Song of the Soul». |
 |
 |
| “Rebti. Portrait of a girl on the background of a waterfall”, artist S. N. Roerich. | |
 |
|
Materials and methods
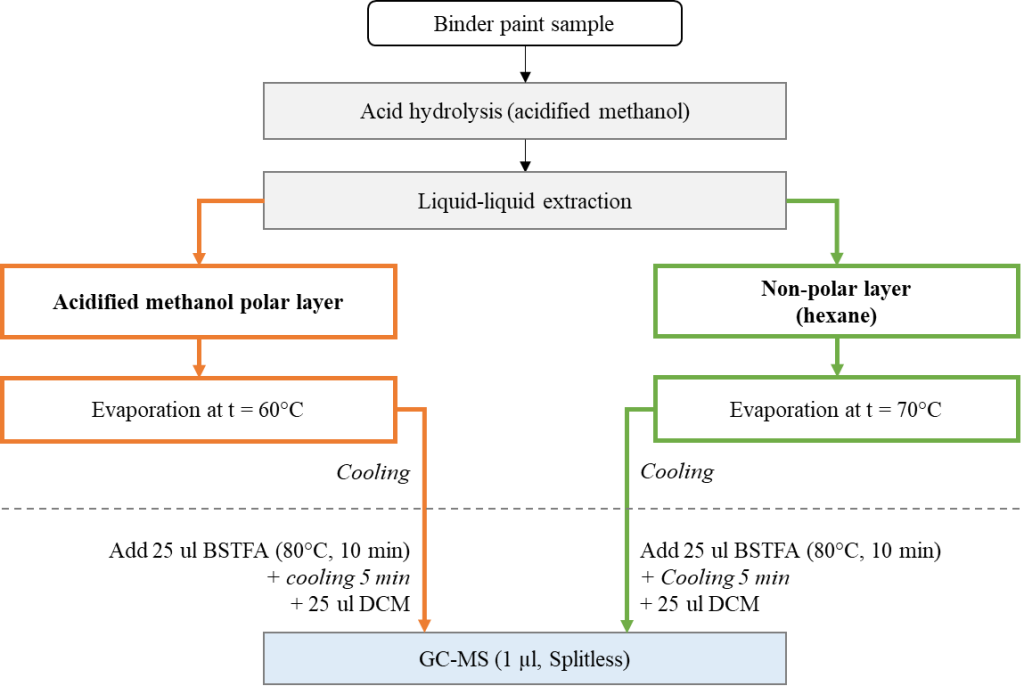
There is a wide variety of methodological approaches to the chromatographic analysis of the organic components of the sample, eventually coming to the use of acid hydrolysis and derivatization [20] of both the inorganic (lipid) and organic fractions of the sample extract. The resulting derivatives of methyl esters (MEGC, FAME) have high volatility properties, and become amenable to GCMS. Other sample preparation protocols found in literature, may involve, in particular, reaction with alkyl chloroformates [5, 6, 21] or transesterification of triglycerides [22-26].
In this study the sample was exposed to acidic methanolysis to form fatty acid methyl esters using an acidified methanol solution (1.2 M HCl/MeOH) followed by hexane extraction. Both the hexane layer and the organic solvent layer were evaporated to dryness then, the dry residues were derivatized with a mixture of N, O-bis – (trimethylsilyl) – trifluoroacetamide (BSTFA) with 1% TMCS (trimethylchlorosilane) at 80°C to produce TMS derivatives of hydroxyacids, alcohols, carbohydrates, and steroids, followed by dilution with dichloromethane.
Hexane, methanol, dichloromethane, concentrated hydrochloric acid (37%) – all reagents used in the experiment were classified as particularly pure or “HPLC grade” quality. As a set of calibration standards, a commercial mixture of fatty acid methyl esters (Nu-Chek-Prep GLC-Nestle36, USA) was used, the purity of each individual substance is not less than 99%. The chromatogram of the standard mixture is shown in Figure 3.
Fig. 6. Scheme of sample preparation, extraction and derivatization of samples during the study.
Instruments and operating modes used in the study
The obtained sample extracts were analyzed with Q-Tek single quadrupole chromatography-mass spectrometry system (Q-Tek, Montenegro). The molecules were ionized in an Electron Impact ion source at 70 eV electron energy and at ion source temperature of 230°C. The experiments used a 30m Mega-5MS chromatographic capillary column (Mega, Italy) ID = 0.25mm and film thickness of 0.25 microns.
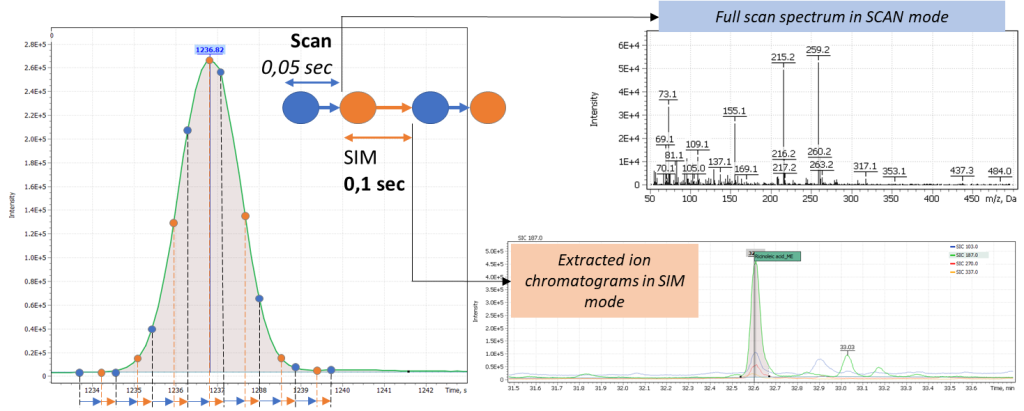
Based on analytical task, the current study used different modes of operation of the quadrupole mass filter. To analyze the fatty acid composition and penta esters of fatty acids, a standard ion scan in the specified mass range (SCAN) was used. For complementary detection of ricinoleic acid traces, a mixed (cyclic) scanning mode (SCAN/SIM) was used, making the mass filter to alternate scanning ions in user specified mass range, with monitoring of selected characteristic ions of ricinoleic acid. The principle of operation of the quadrupole mass filter is shown in Figure 7. The parameters of the chromatographic system and the modes of operation of the mass detector are shown in Table 2.
Fig. 7. The principle of operation of the quadrupole mass filter in SCAN/SIM mode. This approach consists of alternately changing the scanning modes of the mass filter in order to obtain data in a wide dynamic range of concentrations without significant loss of sample information.
Table 2. Parameters of chromatographic separation method.
| Parameters | Non-polar layer
(detection of FAME and DCO) |
Polar layer
(detection of pentaerythritol) |
| GC column | Mega-5MS (30m, 0.25 mm I.D.; 0,25 µм) | |
| Испаритель | Split/Splitless («Single Taper» liner: Restek 4 mm x 6,3 x 78,5) | |
| Volume of injected sample | 1 µL | |
| Split flow program | Splitless, hold 1 min, then 70 mL/min | |
| Inlet temperature | 290°C | |
| Oven program | 60°C/min – 3,0 min;
7.0°C/ min to 200°C – 5,0 min; 7.0°C/ min to 290°C – 30,0 min |
|
| Carrier gas | (Helium) 1 mL\min | |
| GCMS transferline temperature | 280°C | |
| Ion source temperature | 230°C | |
| MSD operating modes | SCAN/SIM | SCAN |
| Scan range | 50 – 350 Da | 50 – 500 Da |
| Characteristic ions selected for methyl ester of ricinoleic acid
(Dwell Time, msec) |
Ion 1 = 187 Da (24 msec)
Ion 2 = 103 Da (24 msec) Ion 3 = 270 Da (24 msec) Ion 4 = 337 Da (24 msec) |
– |
| Solvent delay | 12 min | 10 min |
A preliminary study of the paint binder was carried out using IR microscope LUMOS (Bruker, USA) in the mode of disturbed total internal reflection (NPLR, Ge crystal) in the spectral range from 4000 to 600 cm-1.
Analysis of model objects and commercial paints based on oil binder
Several model objects were used to study, verify and test the characterization method for oil binder of artistic paints. In particular, pure linseed oil from a local pharmacy, samples of fresh paint based on linseed oil binder (Georgian), as well as two samples of paint (dried zinc whitewash) from the laboratory collection (1967). The locations of the paint samples are indicated by the blue arrows in Figure 8.
Fig. 8. Model samples of paints based on vegetable oil binder.
| Oil-based binder paint (Georgian), UK | Zinc whitewash
(specimen #6 and specimen #3), 1967 г |
 |
 |
The obtained Palmitic (C16) to Stearic (C18) acids ratio indices in the model specimens are presented in Table 3. The table shows that all oils used for preparation of binders belong to the type of linseed oil, while, unlike pure linseed oil, a slight increase in the P/S ratio index is observed in the paint. Interestingly, the same relationship was noted in publication of Colombini et al (1999), where it was suggested that white pigment increases the ratio of P/S acids in linseed and poppy oil and, therefore, may be the reason why the index obtained for the analyzed paint samples is slightly higher than in sample of fresh oil [2, 3]. In any case, the values obtained on the model specimens are completely consistent with the literary values of the P/S indices [2].
Table 3. The obtained palmitic (C16) to stearic (C18) acids ratio indices in the model specimens
| Index | Linseed oil | Paint from a tube (Georgian), UK | Zinc whitewash,
model specimen #6 (1967) |
Zinc whitewash,
model specimen #3. (1967) |
Literature Colombini et. al. (1999) [2] |
| P/S | 1.2 | 1.6 | 1.4 | 1.3 | 1.4 |
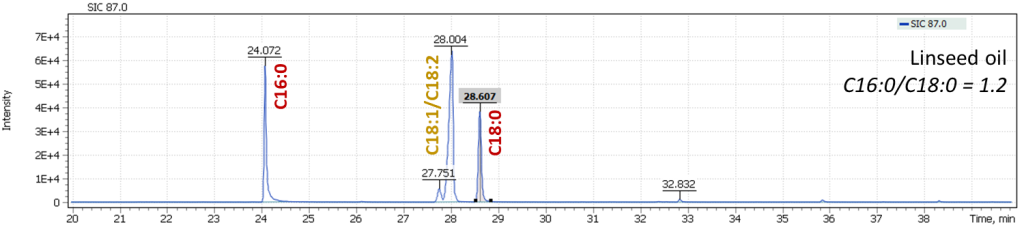
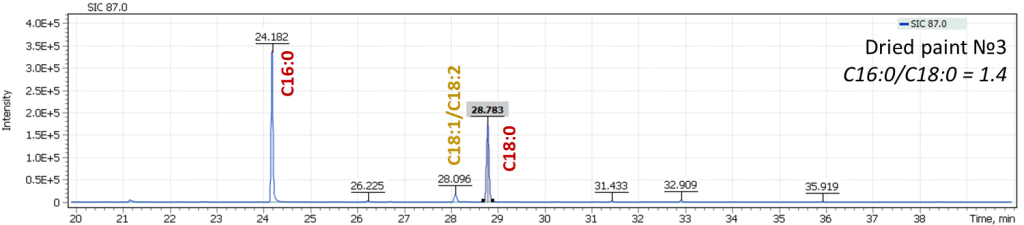
Fig. 9. Mass chromatograms of model specimens extracted for 87 m/z
 |
 |
 |
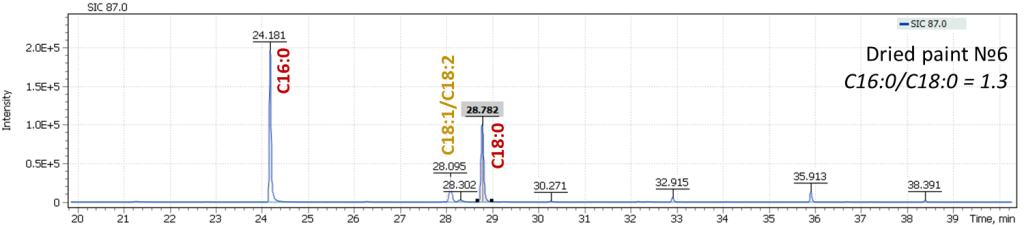 |
In Figure 9, an effect of oils drying is well illustrated, leading to a decrease in content of oleic acid (C18:1) and linolenic acid (C18:2) in the profile, while keeping constant ratio of C16:0 and C18:0 signals, a characteristic of linseed oil. This is another justification for the proposed approach to characterization of oils equally valid both for fresh oils and for the characterization of aged vegetable oil binders. During our work with the model specimens, an analysis was also performed for the presence of dicarboxylic acids formed during the oil aging. Thus, for fresh paints, their signal intensities were at the trace levels or were completely absent, while in aged paints, dicarboxylic acids were confidently detected.
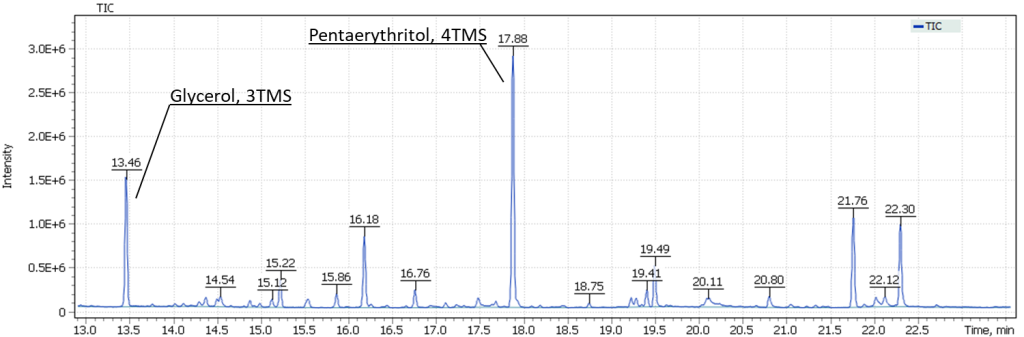
To test method of analysis of pentaerythritol in an oil binder composition, a model specimen of dried penta-oil paint from a tube (lead-zinc whitewash based on a penta-oil binder, LZHK) was used. Figure 10 shows the prepared paint sample, the spectrum of the obtained trimethylsilyl derivative of pentaerythritol, as well as position of the target signal relative to the glycerol signal on TIC chromatogram of the model specimen.
Fig. 10. Model specimen of penta-oil paint (spectrum and position on the TIC chromatogram).
| – model paint specimen | – spectrum of pentaerythritol TMS-derivative |
 |
 |
| – Position on the TIC chromatogram | |
 |
|
Results of the paintings study
The painting ” The Reveller. Oleg Sataev” (V. Zubarev),1972 г.
Painting by V. Zubarev ” The Reveller. Oleg Sataev ” was received for restoration in the Department of Scientific Restoration of easel oil paintings of the State Research Institute of Fine Arts in a very poor state of preservation. The paint layer of the painting was severely damaged in the process of existence, which can be due to several reasons:
- The use of low-quality paints. It is known that the participants of the studio “New Reality”, which included V. Zubarev, were limited in funds, they were not supported by the Union of Artists, so many of them used cheaper sketch and paint paints.
- Violation of the artist’s technology when painting a picture, which is confirmed, for example, by poor adhesion of the paint layers to each other and numerous delaminations (Fig. 11, left).
- The nature of the surface of the paint layer, for example, the folding (shrinkage) of the paint layer (Fig. 11) suggests that the artist could have added siccatives to the paint, speeding up the drying process. In addition, it is possible that this effect was achieved due to a large amount of binder, for example, in Figure 12 (dot #2) the transparent film is visible. It can be assumed that the artist added a binder to the paint and mixed the paint not well enough.
Fig.11. Micrographs of the surface of the paint layer of V. Zubarev’s painting “The Reveller. Oleg Sataev”.
 |
 |
Thus, the preliminary inspection of the artworks details, and first of all – the binder condition, became the main task in order to choose the most suitable restoration method for this painting.
To perform the study, samples of zinc whitewash were taken from the painting. The sampling locations are shown in Figure 12.
- Sample #1 – white paint layer, pigment composition: zinc white.
- Sample #2 – white paint layer with transparent film, pigment composition: zinc white.
Fig. 12. The sampling site in the painting ” The Reveller. Oleg Sataev”, as well as a micrograph of the paint layer surface with sampling sites (#1 and #2).
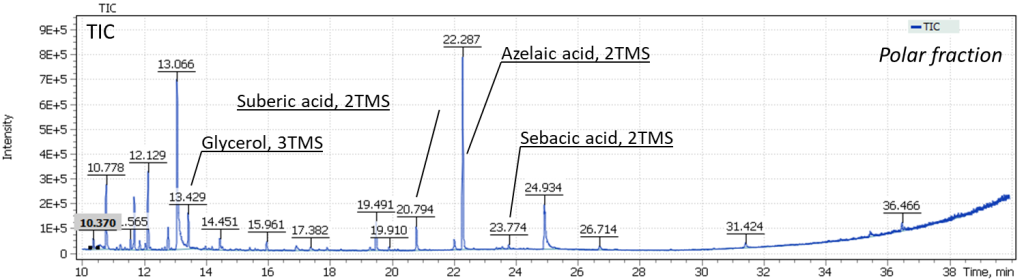
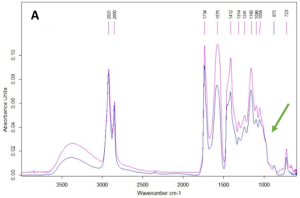
The presence of absorption bands in IR spectra of samples #1 and #2 2921, 2850, 1734, 1241, 1160, 1096, 1054 and 723 cm-1 lead to conclusion that the binding agent of the white paint layer is the oil’s fatty acids pentaerythrite ester (Fig. 13). The absorption band of 1575 cm-1 refers to zinc carboxylates – the product of the interaction of zinc whitewash and binder.
Fig. 13. Study of the white paint layer binder by IR spectroscopy: (A) IR spectra of sampling spots #1 (blue) and #2 (pink), the green arrow indicates the characteristic absorption band of pentaerythritol; (B) photo of sampling spot #2, the red frame indicates the area of the transparent film from where the IR spectrum was recorded.
 |
 |
GCMS analysis was performed to confirm the identification of pentaerythritol. During sample extraction, the target compound was concentrated in the polar fraction of the extract, being carefully evaporated to dryness in a heating block at 60°C. Further, the dry sample residue was derivatized to form trimethylsilyl derivatives. Figure 14 shows the obtained TIC chromatograms of the components from the sampling spots #1 and #2 of the painting.
Fig. 14. TIC Chromatograms of the binder components from sampling spots #1 and #2.
| #1 | 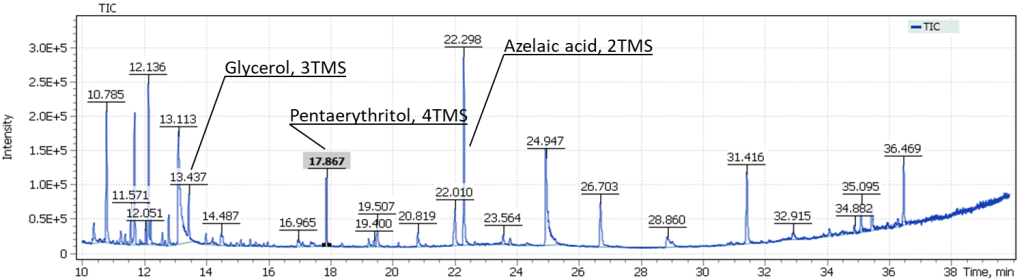 |
| #2 | 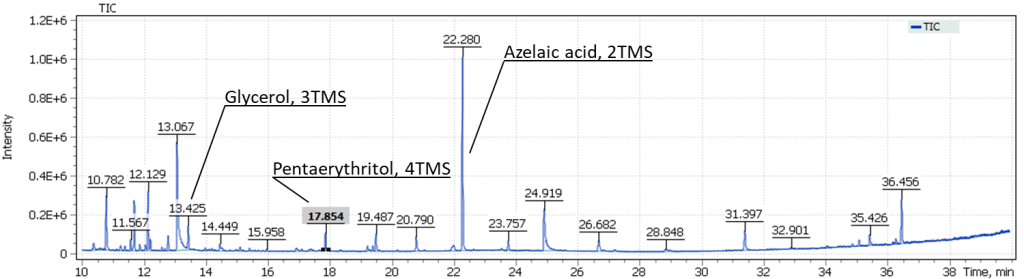 |
The obtained results emphasize complexity of the studied sample composition. The total ion current chromatograms show glycerol signals, as well as the target compound pentaerythritol in both sampling spots #1 and #2.
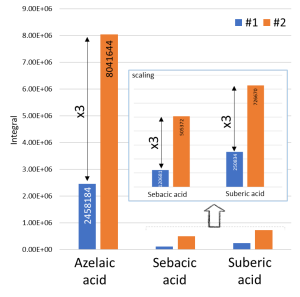
Also in Figure 14, we can distinguish the signals of dicarboxylic acids, in particular, the major signal of trimethylsilyl derivative of azelaic acid. An interesting observation is that the intensity of dicarboxylic acids for the sample taken from spot #2 (the presence of a transparent film) is three times higher than that for the sample taken from spot #1 (Fig. 15).
Fig. 15. Differences in intensity of dicarboxylic acids for samples taken from spots #1 and # 2.
| The content of dicarboxylic acids in the samples | Saturated acid content in samples |
 |
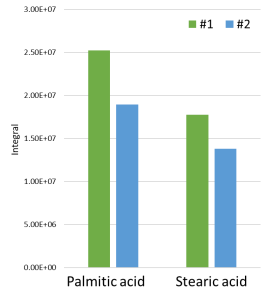 |
At the same time, in order to exclude differences in the initial weight of the analyzed sample, non-polar fraction of the extracts was also analyzed. The study confirmed an approximate similarity in the intensities of methyl esters of palmitic (C16) and stearic (C18) acids, thus suggesting an initial similar weight of the samples. The calculation of the C16/C18 ratio in the binder composition showed that in spot #1, as in spot #2, the presence of linseed oil was detected (P/S = 1.49 for sample #1 and 1.46 for sample #2). The observed difference in the intensity of dicarboxylic acids suggests probable use of several different oil paints (whitewash) or their uneven mixing, resulting in presence of areas with older (aged) oil binders (transparent films).
The painting “Song of the Soul”, author unknown, 1967 г.
The painting “The Song of the Soul” (author unknown) was sent to the Research laboratory of GOSNIIR (Moscow) for forensic expertise. In the lower right corner, there is a signature and date-1967. Examination complexity of paintings dated the second half of the twentieth century comes from the fact that the Soviet paint and varnish industry at that time was already producing a fairly large range of artistic materials, so accurate identification of the composition of both pigments and binders can be a key factor in determining the authenticity of the work.
To perform binding oil GCMS analysis of the painting, a sample of the blue paint layer from the clothes area spot was taken. Preliminary study by polarization microscopy, X-ray spectral microanalysis, and IR spectroscopy revealed the following pigments in the sample: spectral cobalt blue, artificial ultramarine, emerald green, strontian yellow, zinc white, red cadmium sulfide-selenide, red ochre, cobalt green, yellow mars, barite, kaolin, and coal black. The sampling spot location is designated in Fig. 16B with a red border.
Fig 16. Sampling spot of blue paint from “Song of the Soul” painting to perform GCMS analysis of the binder.
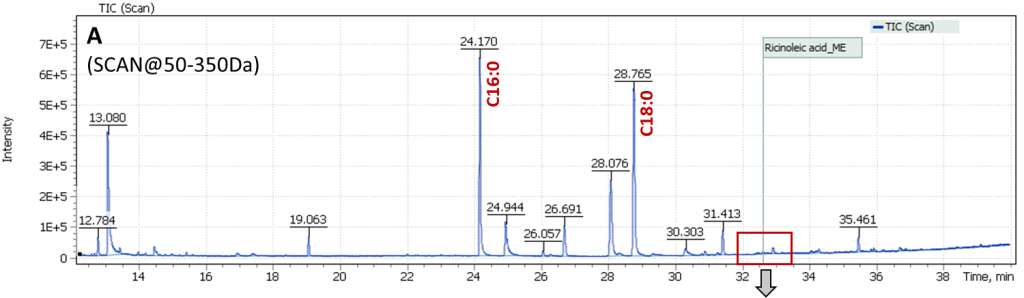
Additionally, preliminary study of the sample using IR spectrometry suggested that dehydrated castor oil (DCO) was used as a binder in the paint layer (Fig. 17). As mentioned above (introduction), production of paints based on such a binder started in the USSR not before the 1980s, which contradicts the date indicated in the picture as 1967. Thus, a detailed and accurate study was necessary to determine the earliest possible date of this artwork creation.
During sample preparation for GCMS analysis, acid methanolysis of the sample material was performed to form fatty acid methyl esters and their subsequent extraction with hexane. The procedure of silylation of evaporated to dryness the hexane extract produced TMS-ether (by the hydroxy group) of the ricinoleic acid methyl ester.
Fig. 17. IR spectrum of the blue paint sample. The green outline shows the absorption region, served as a basis for assumption on DCO presence in the paint composition.
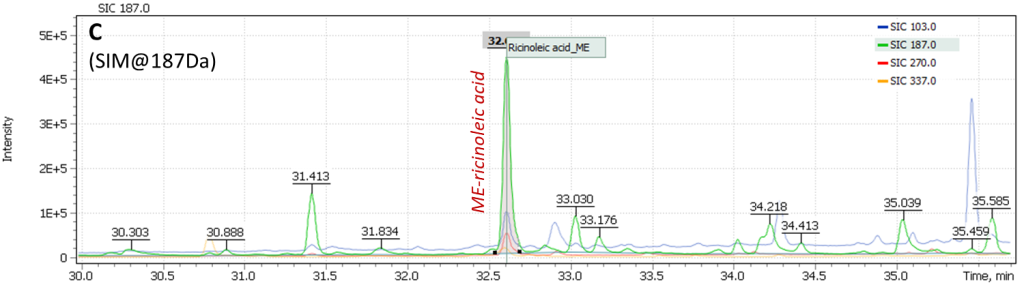
Content of ricinoleic acid in the sample is usually at trace concentration level, so it is necessary to use a quadrupole mass filter in full scan mode combined with selective ion monitoring (SCAN/SIM). The main masses of structural and characteristic ions of TMS-methyl ester of ricinoleic acid are 187.0 Da, 270.0 Da, and 103.0 Da. To set up correct acquisition parameters, the peak of TMS ether is known to elute 0.3 min before arachinic acid C20: 0 peak on the chromatogram (the arachinic acid signal is almost always present in samples of oil binder paints).
Analysis results of the painting “Song of the Soul” are shown in Figure 18: (A) a Total Ion Current chromatogram of nonpolar fraction of the paint extract is presented, (B) an extended section of arachinic acid and ricinoleic acid elution times is presented and Extracted Ion chromatogram (C) shows signal plots of ricinoleic acid TMS-methyl ester characteristic fragment ions collected in SIM mode. The figure clearly shows the signal has high intensity and is reliably detected on the mass chromatogram.
Calculation of palmitic and stearic acids (C16/C18) ratio in the analyzed binder composition results in index P/S = 0.85. Such a low value also suggests the presence of stearates as emulsifiers in the oil binder composition, that needs yet to be confirmed either by means of IR spectroscopy, or by a complementary study for presence of stearates in additionally taken sample (the target compound of the study is methyl stearate).
Fig. 18. Chromatograms of the samples taken from ” Song of the Soul» painting.
Painting by S. N. Roerich ” Rebti. Portrait of a girl on the background of a waterfall», 1936 г.
One direction in painting studies is to establish methods of artists’ work, an “imprint” of their personal technology. The GOSNIIR laboratory is currently working together with the Museum of the East to study paintings of the artist Svyatoslav Roerich, the son of Nicholas Roerich. The study of Roerichs ‘ heritage is of particular interest, since these artists are known to experiment a lot with techniques of painting, and also used rare pigments in their palette.
- Roerich’s painting ” Rebti. Portrait of a girl against a waterfall ” (Fig. 5) was submitted for research in order to elucidate the technique of painting, since various areas of the painting differed in texture and had a matte or glossy surface. Thus, the assumption was put forward about the artist’s work in a mixed technique, that is, the use of several types of binders in one picture.
The study of micro-samples taken from different parts of the painting by IR spectroscopy showed the painting layer was made using drying vegetable oil (Fig. 19). However, as for the purple paint layer from the image of the stones, it was not clear from the results of IR spectroscopy whether there were other binders in the paint, in particular animal protein or egg yolk. This sample was submitted for analysis by gas chromatography-mass spectrometry in order to resolve the following issues:
1) Determine the type of vegetable oil that the artist used in his work (the main drawback of the IR spectroscopy method, which is routine when analyzing the binder of paintings — is inability to determine the type of oil (linseed, poppy or nut), which significantly restricts researchers in studying technological features of the artists ‘ work).
2) Detect presence or absence of animal origin compounds in the purple color layer (for example, cholesterol).
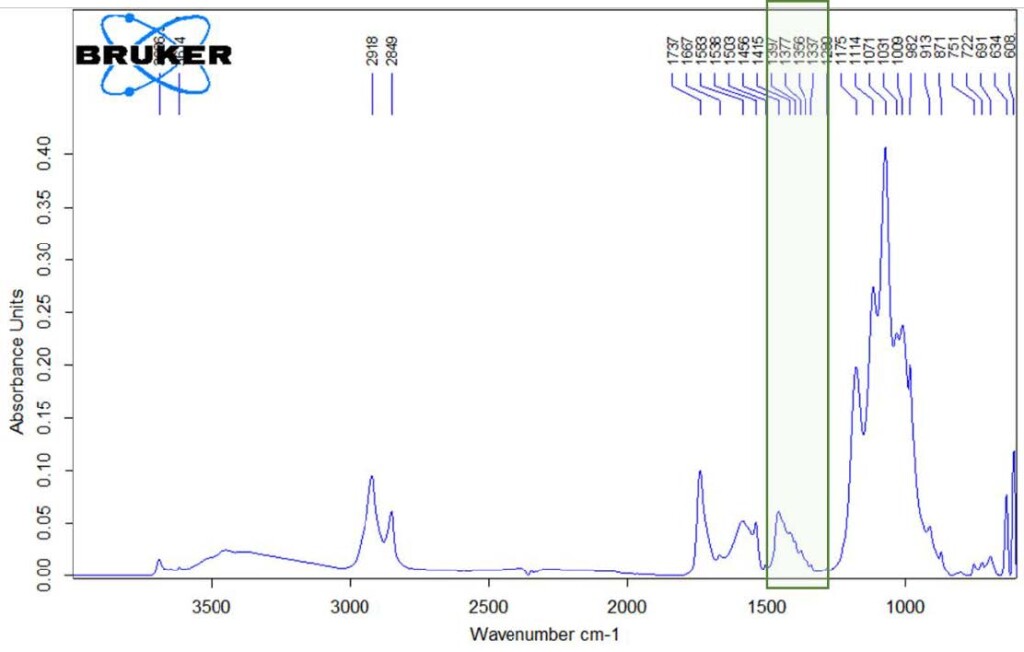
Fig. 19. IR spectrum of a purple paint sample from the image of boulders in the painting “Rebti. Portrait of a girl on the background of a waterfall”, S. N. Roerich.
The absorption in the region of 2919, 2850 and 1730-1700 cm-1 suggests presence of drying vegetable oil. However, the characteristic absorption region of ester groups in the oil (1250-1000 cm-1) is blocked by intense and wide absorption due to presence of artificial ultramarine in the sample (absorption band 991 cm-1). The green outline in Figure 19 shows an absorption region that is not related to vegetable oil, and it was assumed that this region is associated with the presence of a protein origin compound.
Sample preparation for GCMS analysis was carried out as to obtain two fractions, where the non – polar fraction was used to analyze fatty acid composition of the sample and characterize the oil, and the polar fraction was used to detect alcohols and to check for presence of fatty acid pentaesters. To ensure complete dissolution of the sample, methylation procedure with the reaction mixture was carried out for 8 hours in a heating block at temperature of 80°C and then the mixture was left overnight at room temperature. Completeness of the dissolution was monitored visually.
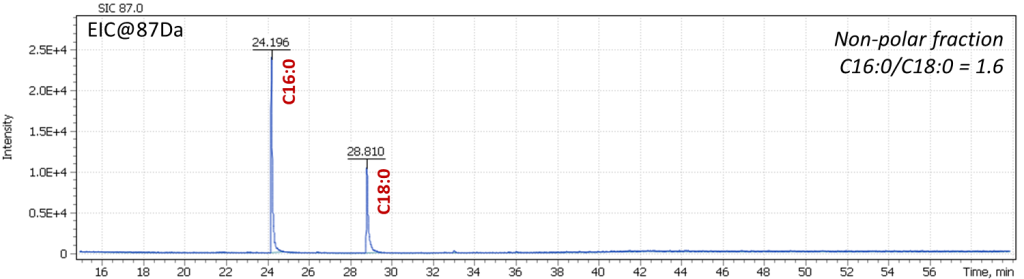
Figure 20 shows chromatograms of the obtained extracts. The stearic and palmitic fatty acids ratio (AC@87 Da) was P / S = 1.6, which corresponds to the composition of the linseed oil binder. The purity of the mass chromatographic profile and the absence of signals of oleic and linolenic acids were noted as an indication of the proper binder age in the analyzed sample.
Fig. 20. TIC Chromatograms of polar (top) and nonpolar (bottom) fractions of the sample extracts of the painting “Rebti. Portrait of a girl on the background of a waterfall”, by S. N. Roerich.
Presence of aged oils was also demonstrated in the results of the analysis of the organic layer of the sample. High signal intensities of dicarboxylic acids are formed during self-oxidation of polyunsaturated acyl chain present in the binding oils and is a marker of their aging completeness. Signal of polycyclic alcohol cholesterol, as a marker of the presence of egg yolk, was not detected in the binder sample.
Conclusion
Dating study of painting artworks should be based only on laboratory examination of paint layer and ground materials. An expertise that does not delve into all the “little things”, or pay little attention to morphological features of pigments, or does not identify binder composition, is prone to misleading conclusions. The experts in the restoration and evaluation of paintings should be encouraged to introduce new means and methods of study in order to conduct proper research to confirm the hypotheses put forward.
The GCMS method allows to reliably identify the type of oil binder based on plant origin, as well as new types of binders based on pentaerythrite esters of sunflower oil fatty acids (“penta-oil paints”) and based on dehydrated castor oil (DCO).
The chemical industry does not stand still and continues to produce more and more complex paint compositions. Consequently, experts are forced to keep research and interpretation of new emerging paint compositions in forensic examination of the artworks. Only detailed characterization of paints composition along with search for certain markers will allow in future to reliably confirm the value of paintings, as well as choose optimal restoration technology.
This paper presents use of GCMS method in forensic expertise of artworks. In particular, to choose right restoration strategy (V. Zubarev’s painting), to confirm authenticity of the paintings (the painting “Song of the Soul”), as well as typical GCMS use for analyzing the type of oil binder and, accordingly, determining painting technique of the artist (Roerich).
This work is expected to continue and expand the GCMS method into area of the art history, in particular, for search of marker substances to be found in composition of paint layers, soil, canvases and paintings.
Literary references
- Ю.И.Гренберг. Краски ХХ в. и экспертиза произведений русской масляной живописи.
- Colombini, M. P., Modugno, F., Giacomelli, M. & Francesconi, S. (1999). Characterisation of proteinaceous binders and drying oils in wall painting samples by gas chromatography–mass spectrometry. Journal of Chromatography A, 846 (1): 113-124. doi: https://doi.org/10.1016/S0021-9673(99)00344-1.
- Manzano et al. / Talanta 84 (2011) 1148–1154.
- S. Mills, Stud. Conserv. 11 (1966) 92.
- Peris-Vicente, J.V. Gimeno Adelantado, M.T.D. Carbo, R.M. Castro, F.B. Reig, J.Chromatogr. A 1101 (2006) 254.
- Lluveras, I. Bonaduce, A. Andreotti, M.P. Colombini, Anal. Chem. 82 (2010) 376.
- Kouloumpi, G. Lawson, V. Pavlidis, Anal. Bioanal. Chem. 387 (2007) 803.
- Bonaduce, M. Cito, M.P. Colombini, J. Chromatogr. A 1216 (2009) 5931.
- Andreotti, I. Bonaduce, M.P. Colombini, G. Gautier, F. Modugno, E. Ribechini, Anal. Chem. 78 (2006) 4490.
- S. Mills, R. White, The Organic Chemistry of Museum Objects, Butterworth-Heinemann, Oxford, UK, 2006.
- Singer, R. McGuigan, Ann. Chim. (Rome, Italy) 97 (2007) 405.
- K. Tsakalof, K.A. Bairachtari, I.D. Chryssoulakis, J. Sep. Sci. 29 (2006) 1642.
- Colombini, M. P., & Modugno, F. (2004). Characterisation of proteinaceous binders in artistic paintings by chromatographic techniques. Journal of separation science, 27(3), 147P
- Organic mass spectrometry in art and archaeology / ed. by Colombini M.P., Modugno F. – Oxford: Wiley-Blackwell, 2009. – 493 p.: [8] p. of plates: ill. – Incl. bibl. ref. – Ind.: p.483-493. – ISBN 978-0-470-51703-1.
- Shilling M.R., Khanjian H.P., Gas chromatographic determination of the fatty acid and glycerol content of lipids. I: The effects of pigments and ageing on composition of oil paints, ICOM Committee for Conservation Preprints 11th Triennial Meeting, James and James, London, 1996: 220–227.
- Colombini M.P., Fuoco R., Modugno F., Menicagli E., Giacomelli A., GC-MS characterization of proteinaceous and lipid binders in UV aged polychrome artefacts, Microchemical Journal, 2000, 67, 291–300.
- С.Д. Колюбакин. Государственный Эрмитаж. Газохроматографическое определение синтетических связующих второй половины XX века в произведениях станковой масляной живописи.
- М. М. Девятов. Новый метод изготовления художественных масляных красок. «Сообщение Всесоюзной центральной научно-исследовательской лаборатории по консервации и реставрации музейных художественных ценностей». М., 1963, № 7, стр. 6—19.
- А. М. Никитин. Художественный краски и материалы. Справочник. – М.: Инфра-Инженерия, 2016. – 412 с.
- Eras, F. Montanes, J. Ferran, R. Canela, J. Chromatogr. A 918 (2001) 227.
- P. Colombini, F. Modugno, E. Menicagli, R. Fuoco, A. Giacomelli, Microchem. J. 67 (2000) 291.
- Sutherland, J. Chromatogr. A 1149 (2007) 30.
- Piccirillo, D. Scalarone, O. Chiantore, J. Anal. Appl. Pyrol. 74 (2005) 33.
- M. Challinor, J. Anal. Appl. Pyrol. 61 (2001) 3.
- Dron, R. Linke, E. Rosenberg, M. Schreiner, J. Chromatogr. A 1047 (2004) 111.
- Watts, E.R. de la Rie, Stud. Conserv. 47 (2002) 257.
- Григорьева И. А. Применение ИК-спектроскопии для анализа связующих красок в произведениях масляной живописи, С. 141–174. – в книге: Ю. Гренберг, С. Писарева Масляные краски XX века и экспертиза произведений живописи. Состав, открытие, коммерческое производство и исследование красок. М., 2010