Shakhov А. V. Head of lab
“EFCO Food Ingredients “
Koluntaev Dm. Ph.D in biology,
Q-Tek d.o.o., Bar, Montenegro
Chuvelev E.
Q-Tek d.o.o., Bar, Montenegro
Summary
The Q-Tek GC-MS chromato-mass spectrometry system performance was demonstrated by quantitation of residual amounts of glycidyl esters of fatty acids in vegetable oil samples.
Introduction
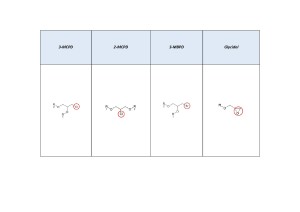
Esters of 3-monochloropropanediol (3-MCPD) and glycidyl esters of fatty acids are food impurities that can be found in small amounts in vegetable oils. But even small quantities can exhibit genotoxic effects, which were scientifically justified in the 83rd report of the joint FAO/WHO Expert Committee on food additives “Conclusion on the presence of certain impurities in food” and in the Conclusion of the European Food Safety Agency group on impurities in the food chain (CONTAM) “Risks to human health associated with the presence of 3-and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl esters of fatty acids in food ” (Fig. 1).
The principle of their action is that the glycidyl esters of fatty acids are hydrolyzed in the gastrointestinal tract to glycidol, which, in turn, is a genotoxic compound. In May 2016, the European Food Safety Authority (EFSA) published a report that shows the relationship between eating palm oil refined at temperatures above 200°C and the likelihood of developing cancer. Previously, experts from the World Health Organization (WHO) have also highlighted this relation. Since 2018, the European Union has introduced laboratory control of residual amounts of glycidyl esters in food products, as well as approved norms for their presence. Not so long ago, Russian Federation also started talking about the need to control the content of glycidyl esters of fatty acids. The oil and fat industry have accepted this challenge and major manufacturers have joined the actions to mitigate the problem. EFCO Food Ingredients Group of Companies was able to measure by use of Q-Tek GC-MS instrument and subsequently reduce the content of glycidyl esters in its products.
The source of fatty acids glycidyl esters formation can be a deviation from refining technology for vegetable oils, in particular, when heating the oils to temperatures exceeding 200°C, during deodorization and / or transesterification stages.
There are several methods for controlling glycidyl esters of fatty acids: the method involving slow acid esterification of vegetable fat in order to remove the matrix, transfer of glycidyl esters to monobrompropanediol and, then, obtaining esterified forms with phenylboric acid, as well as the method of slow alkaline esterification. This paper involves slow acid esterification method, (AOCs Cd 29a, (b, c) – 13, ISO 18363-3, and DGF CIII 17(18) (09) B methods).
Fig. 1. Structural formulas of the analyzed compounds.
Experimental
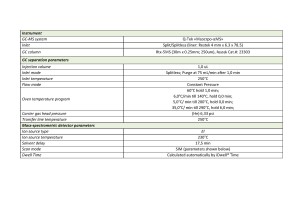
In an acidic solution containing bromides, glycidol esters are converted to 3-monobromopropandiol monoesters (3-Mbrpd), after which 3-MBrPD esters together with 3-MCPD 2-MCPD esters pass into a free (non-esterified) form in an acid solution containing methanol. Next, the step of removing methyl esters of fatty acids is performed, after which the glycidyl esters of fatty acids formed during the reaction are extracted from the sample and derivatized using phenylboric acid. Upon completion, the extract is injected into a gas chromatograph with a mass spectrometric detector, operated in scanning selective ions (SIM) mode with data acquisition parameters listed in Table 1 and 2 below.
General scheme of the method
Table. 1. Data acquisition method parameters.
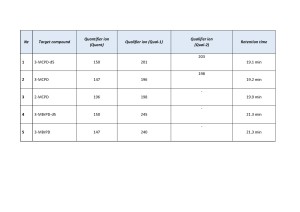
The list of target compounds and their detection parameters are shown in Table 2.
Table 2. Elution times of target ions’ peaks and their scanning parameters.
Fig. 2. Model chromatogram of standard mix of glycidyl esters in the oil matrix (at their concentrations of 0.5 ppm).
- 3-MCPD-d5; 2. 3-MCPD; 3. 2-MCPD; 4. 3-MBrPD-d5; 5. 3-MBrPD
Flexible SIM post-processing algorithm.
The vegetable origin matrix, in particular oil, is a complex biological object rich of high-molecular organic compounds. Their dense population and intensity in the spectrum form a so-called “spectral matrix”, which often does not allow reliable detection of target ion signals. This effect is expressed by the distortion of the ion signal level and the inability to achieve low detection limits of the method. The flexible SM post-processing algorithm, a built-in productivity tool of Q-Tek Analyst software package, helps to avoid this problem. The algorithm operation has been described in detail before [12]. In short, the algorithm allows operator to change the quantifier ion used for system calibration after data acquisition and then do quantitative analysis (“post-process”).
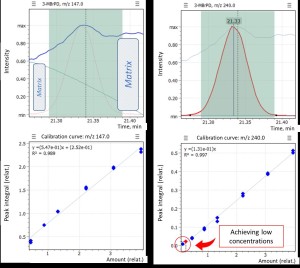
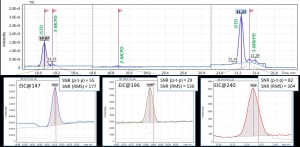
Figure 3 shows a matrix calibration for the 3-MBrPD compound, where you can notice overlap on target signal at low concentrations (m/z 147 Da) due to matrix interference. The use of the “Flexible SIM” algorithm made it possible to quickly switch to using the qualifier ion as the new quantifier free from interference and correctly build the calibration curve for the desired compound (3-MBrPD) over the entire range of required concentrations.
Fig. 3. Matrix calibration of the 3-MBrPD compound showing signal overlap (@ m/z=147) at a concentration level of 0.069 ppm due to “matrix” interference.
Results and discussion
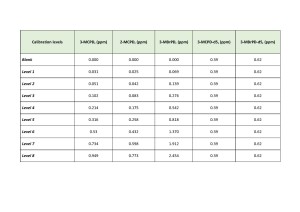
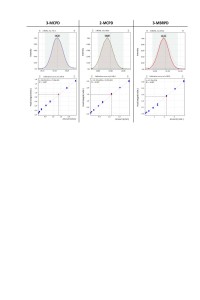
Quantitative determination of fatty acid glycidyl esters was performed using internal standard calibration. Deuterated standards for defined components were used as internal standards. To build the calibration dependence, 9 calibration levels were used, including “blank” (table. 3). The correlation coefficients for all the target compounds of fatty acid glycidyl esters were R2 ≥0.99 (Fig. 4).
Table 3. Calibration levels for 3-MCPD, 2-MCPD, and 3-Mbps compounds, as well as their deuterated internal standards.
(all values are adjusted for the purity of the standard substances used).
Fig. 4. Linear calibrations and ion current signals (quantifiers) for the target analytes.
Approbation Method
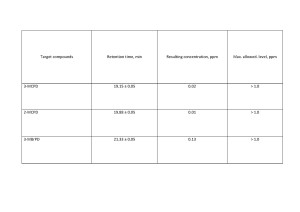
This method has been tested on various samples of oils, fats, and fat-and-oil substances. Figures 5 and 6 show chromatograms of sunflower oil and corn oil. The results of the study are presented in tables 4 and 5.
Results with a high degree of extraction (87% – 108%) were obtained for all samples and components. The reliability and repeatability of the results obtained are noted as satisfactory. The use of internal standards and the use of matrix calibration to build a calibration relationship play an important role for this kind of sample. All these actions aimed to minimize possible “matrix effect”, led to high accuracy of the results obtained. It should be noted that the method is applicable for both solid and liquid fats and oils.
The linearity of the method was observed over the entire concentration range from 0.03 to 0.93 ppm. The limit of quantitation of the method (LOQ) was not less than 0.05 ppm, and the instrument detection limit (LOD) was not less than 0.01 ppm.
Fig. 5. TIC chromatogram of the sunflower oil sample.
Table 4. The results of the analysis of sunflower oil.
Fig. 6. TIC chromatogram of corn oil sample.
Table 5. The results of the analysis of corn oil.
Conclusions
The relative simplicity of the method and the stability of the results make it possible to control the content of fatty acid glycidyl esters in the incoming raw oils and fat-and-oil mixtures, as well as in the intermediate products at various production stages of the oil. The method can be used for optimizing the production process and technologies in order to increase yield of high-quality food products.
Q-Tek GC-MS chromato-mass spectrometry system allows detecting fatty acid glycidyl esters at concentration levels sufficient for reliable control of their residual amounts in samples as per the relevant regulations in EU, Russia and other countries.
Literature
- Kraft R., Brachwitz H., Etzold G., Langen P., Zöpel H.-J. Massenspektrometrische Strukturuntersuchung Stellungsisomerer Fettsäureester der Halogenpropandiole (Desoxyhalogen-glyceride). J. Prakt. Chem. 1979, 321 pp. 756–768.
- Ermacora A., & Hrnčiřík K. A Novel Method for Simultaneous Monitoring of 2-MCPD, 3-MCPD and Glycidyl Esters in Oils and Fats J. Am. Oil Chem. Soc. 2013, 90 pp. 1–8.
- Ermacora A., & Hrnčiřík K. Evaluation of an Improved Indirect Method for the Analysis of 3-MCPD Esters Based on Acid Transesterification J. Am. Oil Chem. Sos. 2012, 89 pp. 211-217.
- ISO 18363-3:2017 Animal and vegetable fats and oils — Determination of fatty-acid-bound chloropropanediols (MCPDs) and glycidol by GC/MS — Part 3: Method using acid transesterification and measurement for 2-MCPD, 3-MCPD and glycidol.
- Karel Hrncirik, Unilever, The Netherland Indirect methods for the Analysis of MCPD and Glycidyl Esters: A Critical Overview.
- Deutsche Gesellschaft für Fettwissenschaften: DGF Einheitsmethode C-VI 17 (10) Abteilung C 1. WVG Stuttgart (2010).
- Deutsche Gesellschaft für Fettwissenschaften: DGF Einheitsmethode C-VI 18 (10) Abteilung C 1.WVG Stuttgart (2010).
- Miyazaki K, Koyama K, Sasako H, Hirao T: Indirect Method for Simultaneous Determinations of 3-Chloro-1,2-Propanediol Fatty Acid Esters and Glycidyl Fatty Acid Esters. J Am Oil Chem Soc 89, 1403–1407 (2012).
- International Agency for Research on Cancer: Monographs on the Evaluation of Carcinogenic Risk to Humans. Vol. 77. Some industrial Chemicals. monographs.iarc.fr/ENG/Monographs/vol96/index.php (letzter Zugriff: 09.01.2013).
- AOCS Official Method Cd 29a-13 2- and 3-MCPD Fatty Acid Esters and Glycidol Fatty Acid Esters in Edible Oils and Fats by Acid Transesterification.
- Crews C, Chiodini A, Granvogl M, Hamlet C, Hrncirik K, Kuhlmann J, Lampen A, Scholz G, Weisshaar R, Wenzl T, Jasti PR, Seefelder W: Analytical Approaches for MCPD esters and Glycidyl esters in food and biological samples: A review and future perspectives. Food Addit Contam A 30,11–45 (2013).