Keywords:
GC-MS, museum varnishes compositions, resins, drying plant oils
Abstract
Selecting a proper analytical approach for determining the compositions of museum varnishes is an important step for choosing a right method of work-of-art restoration since the effect environmental conditions have on a varnish surface make it the most vulnerable part of an artwork.
As for their composition, varnishes are resins consisting of a number of similar chemical compounds dissolved in different drying oils. GC-MS has been the most applicable analytical solution to study museum varnishes that enables one to determine the biomolecular markers characterizing a resin’s botanic origin and used drying oil.
In the present study, an investigation of commercially available natural resins of known botanic origin was performed to describe their complex characteristics. The suggested analytical approach was tested to investigate varnish samples from two artworks from the Qajar-dynasty arts collection.
Experimental
To develop the procedure for natural resin marker extraction, it was suggested to use a number of reference samples such those of rosin, mastic and dammar that were obtained from the collection of the Research Institute for Restoration. A sample of manila resin was purchased in an art restoration store.
The extraction of target compounds and their purification are important steps in developing an analytical method. They become especially crucial when it comes to working with artworks for, in this case, only a very small sample can be taken, so the applied extraction techniques are to be effective and, if possible, universal. In this study, the following universal sample preparation technique was applied: an extracted sample underwent acid methanolysis to produce FAME (1,2M HCl/MeOH) that were extracted by hexane. The hexane layer was dried at 70°C and the residues were derivatized using BSTFA with 1% TMCS at 80°C to obtain TMS-derivatives. All the compounds used in the experiment such as hexane, methanol, DCM, HCl and all derivatizing agents were of analytical grade.
To perform the analysis a GC-MS (Q-Tek, Montenegro) GC-MS system was used. Depending on a task the different acquiring modes of the quadrupole mass filter were enabled, e.g., to analyze the reference samples, the standard scanning mode (50-500 Da) was set. To detect the biomarkers’ trace amounts a mixed (cycled) scanning mode (SCAN/SIM) was enabled. In this mode, the mass filter not only detected ions in a wide mass range (50-500 Da) but also selected characteristic ones (SIM). This approach to scanning allowed not only detecting the presence of resin biomarkers in the samples but also characterizing the type of drying oil used to prepare a varnish. The list of the biomarkers’ characteristic ions (for SIM mode) can be seen in Table 1. Solvent delay time was set at 6.5 min.
Helium was used as a carrier gas at the flow rate of 1.0 ml/min. 1µl-sample was injected into the GC inlet (splitless mode). The used liner was Single Taper type (Restek 4 x 6.3 x 78.5 mm). To separate the target components, an Rtx-5MS column was used (30 m × 0.25 mm, 0.25 μm). The GC oven was programmed as follows: initial temperature of 60°C (1 min); heating to 280°C at 15°C/min; keeping at 280°C for 1 min. The transferline temperature was set at 250°C, and the ion source’s – at 230°C.
The experiment involved varnish samples taken from 2 museum objects: a book cover (Fig 1A) and a chest lid (1B) from the Qajar-dynasty arts collection of the State Museum of Oriental Art. At that time, the objects were being investigated at Research Laboratory of Physical and Chemical Studies to detect the material and describe the techniques the Iranian craftsmen used in their work.
Results and Discussion
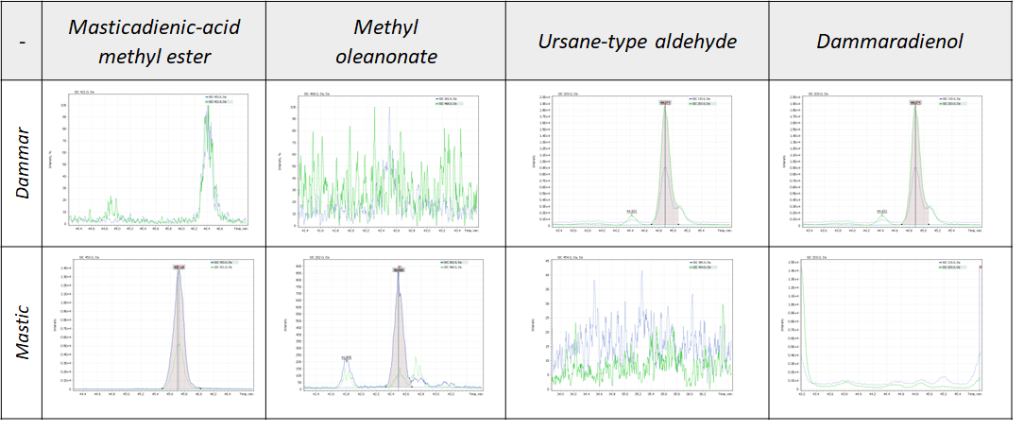
At the first stage of the investigation the analysis of reference resin biomarkers was performed. In our experiment, the mass spectrum of the components of a reference dammar sample was interpreted according to published data (Colombini et al., 2010; Akiyama et al., 2010). In addition, a system of linear retention indices in combination with NIST library (ver. 2020) were applied. The molecular structures of some of the detected components can be seen in Fig.2. According to the results, the sample is mainly composed of dammaradienon, dammaradienol, ursane-type aldehyde and oleanolic acid derivatives.
Figure 2. TIC of derivatized dammar extract sample.
Further investigation demonstrated that analogous dammarane compounds can also be found in mastic resin, but unlike dammar it contains a higher concentration of oleanolic compounds, which is consistent with the published data (Colombini et al., 2010). Hence, the experiment has made it possible to detect the biomarkers to distinguish dammar from mastic resins (Fig. 3).
Figure 3. Biomarkers to distinguish dammar and mastic resins.
Rosin is a solid residue obtained through distillation of turpentine balm extracted from different palm trees. It contains 90% of fee isomeric resin acids (C20H30O2) and small amounts of resins (С20-, С25– and С30– hydrocarbons) and resinols as well as fatty acids. Abietinic and pimaric acids are the most stable resinol isomers. Being of similar composition, they have two unsaturated bounds easing their oxidation. As a result, rosin in time gets less solvable and brownish.
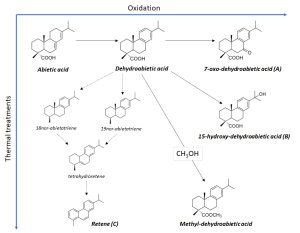
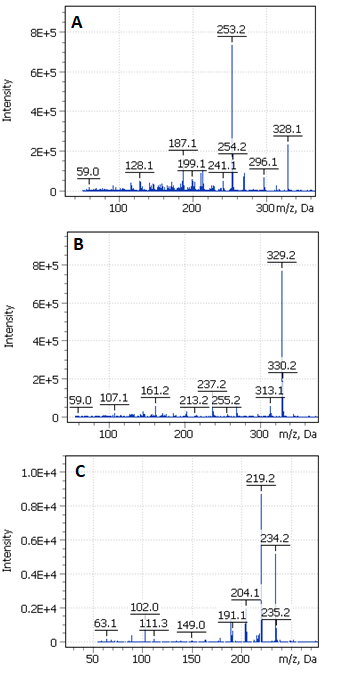
Their origin is not the only parameter to tell rosins apart. According to the literature, the main component of French rosin is pimaric acid, and that of American rosin is abietinic acid. It is also noteworthy that rosin is often added into more expensive resins such as shellac, dammar, sandarac, which, on one hand, cheapens the final product but, on the other, reduces the quality of obtained varnishes (Slansky, 1962). The characteristic compounds that presented in the reference rosin sample can be depicted as an oxidation and thermal decomposition processes scheme (Fig. 4). According to Colombini & Modugno, 2008, such processes are characterized by either dehydroabietic acid production and its further transformation into a number of intermediate products down to retene (thermal effect) or by production of the acid’s derivatives such as 7-oxo-dehydroabietic acid and 15-hydroxy-dehydroabietic acid (oxidation effect). The presence of methylated diterpenoid acids may be a sign that a rosin has been obtained through pyrolytic processing of a pine’s resinous wood when the methanol released while heating reacts with diterpenic acid (see Fig. 4). The product of such a reaction is, first hand, methyl dehydroabietate that absent in the resin (Colombini, Modugno, 2008; Colombini et al., 2005; Pollard, Heron, 1996). In the right part of Fig. 4, you can find the spectra obtained while investigating the rosin extract and see that they correspond to compounds A, B and C in the scheme. It is apparent that the presence of certain biomarkers in a sample’s final extract may enable one to determine the way how a certain kind of varnish was prepared.
The results of research into every reference resin sample can be seen in Table 1 that presents the set of ions for individual biomarkers that has allowed developing a high-sensitivity method for identification of different kinds of resins used in museum varnishes.
Figure 4. Characteristic compounds present in rosin extract (Colombini, Modugno, 2008).
Table 1. Biomarkers and their structural characteristic ions.
| Biomarkers | Type of resins | Main ion | Confirming ion | RT, min |
| Retene | Rosin | 219 | 234 | 21.54 |
| Methyl 6-DHA | Rosin | 237 | 312 | 23.00 |
| Methyl-DHA | Rosin | 299 | 314 | 23.07 |
| Methyl abietate | Rosin | 241 | 256 | 23.75 |
| 15-Methoxy-DHA, ME | Rosin | 269 | 329 | 25.09 |
| Labda-8(20),13-dien-15-oic acid, 19-hydroxy-, ME, (E)- | Copal | 203 | 302 | 25.66 |
| 7-Oxo-DHA, methyl ester | Rosin | 253 | 328 | 25.95 |
| Labda-8(20), 13-dien-15,19-dioic acid-di(TMS) ester | Copal | 307 | 189 | 26.97 |
| Dammarenolic acid ME | Dammar | 385 | 454 | 34.89 |
| Dammaran-3-one, 20,24-epoxy-25-hydroxy | Dammar | 143 | 399 | 42.20 |
| Methyl oleanonate | Mastic | 189 | 262 | 42.51 |
| Methyl moronate | Mastic | 409 | 143 | 42.79 |
| Ursolic aldehyde | Dammar | 203 | 133 | 44.89 |
| Masticadienonic acid ME | Mastic | 453 | 421 | 45.72 |
Varnish samples from the Qajar-dynasty arts collection
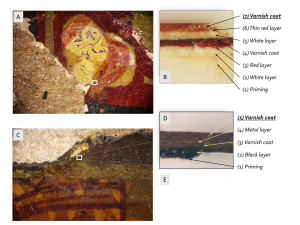
The devised analytical method was applied to analyze the two varnish samples. In Fig. 5, you can see the places where the samples were taken from the book cover/chest lid as well as their paint layers. For the GC-MS analysis, samples were taken from layer 7 (Fig. 5, A, B) of the book cover (Sample 1), and from layer 5 (Fig 5 C-E) of the chest lid (Sample 2).
The analysis results are presented in Fig. 6, part A showing a combined TIC and mass chromatograms (SIC) for structural characteristic ions, and Part B – the detailed target mass peaks. The presence of specific biological markers such as abietic acid derivatives enabled us to conclude that the varnish from Sample 1 was rosin.
Figure 5. Fragments of painted layers from the book cover (A) and chest lid (C) and sampling sites.
B, D, E are the layers’ cross-sections.
Figure 6. Combined chromatograms for structural characteristic ions (Sample 1).
One of the most common approaches to analytical determination of an oil component has been considering the ratio of palmitic to stearic acid in extract (P/S) (Mills, 1977; Andreotti et al., 2006), which does not depend on varnish aging and serves as an ideal marker to identify a drying plant oil (Manzano et al., 2011). Its P/S ratio comprised 1.78 marking the presence of linseed oil as a basic component, which has been confirmed by literature sources mentioning linseed oil as a part of the formulas for varnish preparation in the Middle East (Armenini, 1977).
According to the obtained GC-MS results, samples 1 and 2 had quite similar compositions. In Sample 2, mainly abietic acid derivatives were detected. Its P/S ratio was 1.64 that also indicates that while preparing the varnish, they dissolved rosin in linseed oil too (Fig. 7).
Figure 7. Combined chromatograms for structural characteristic ions (Sample 2).
Additionally, ratios of abietic acid derivatives contents were calculated for both samples for the purposes of future estimation of possible storage condition effects. To do so, every detected biomarker signal was integrated at its peak to estimate its relation to the sum of all abietic acid derivative integrals. The obtained results have made it possible to conclude that the high content of retene in Sampe 1 indicated that the varnish was prepared for a long time and at high temperatures. The high content of 15-methoxy-dehydroabietic acid in Sample 2 suggested the chest had not been kept in appropriate storage conditions.
Conclusion
The main objective of the present study was to develop and test a GC-MS method for determining the composition of a varnish made of natural resins for restoration purposes. Analysis of reference samples have made us possible to detect the characteristic biomarker groups and differentiate kinds of varnishes based on their botanic origin.
The suggested method was tested in two varnish samples obtained from Qajar-dynasty antiques. It has been demonstrated that the Iranian craftsman of the 19th century used rosin-based varnish melted in hot linseed oil. Based on the obtained results, suggestion have been made about the method of rosin extraction and the effect of storage conditions on the museum object in question.
References
- Colombini MP., Modugno F. Organic mass spectrometry in art and archaeology. Oxford: Wiley-Blackwell, 2009. – 493 p.: [8] p. of plates: ill. – Incl. bibl. ref. – Ind.: p.483-493. – ISBN 978-0-470-51703-1
- M. Pollard, C. Heron. Archaeological Chemistry; RSC Paperbacks, Cambridge, 1996.
- Colombini MP, Modugno F, Ribechini E. Direct exposure electron ionization mass spectrometry and gas chromatography/mass spectrometry techniques to study organic coatings on archaeological amphorae. J. Mass Spectrom., 40, 675–687, 2005.
- Rene de la Rie E. Old Master Painting. Analytical Chemistry, Vol. 61, No, 21, November 1, 1989.
- Dietemann P. Towards more stable natural resin varnishes for paintings the aging of triterpenoid resins and varnishes. A dissertation submitted to the Swiss Federal Institute of Technology (ETH) Zurich, 2003
- Armenini GB. On the True Precepts of the Art of Painting; Burt Franklin & Co.: New York, 1977.
- Mills JS. Stud. Conserv. 11 (1966) 92.
- Andreotti A, Bonaduce I, Colombini MP, Gautier G, Modugno F, Ribechini E. Anal. Chem. 78 (2006) 4490.
- Manzano et al. / Talanta 84 (2011) 1148–1154.
- Mills JS, White R. Stud. Conserv. 1977, 22, 1231.
- Slansky B. Painting techniques. – USSSR Painting Academy, М., 1962 (In Russian).
- Colombini et al. Strategies for Characterizing Organic Paint Media, Accounts of chemical research, June 2010, Vol. 43, No.6, 715-727.
- Akiyama et al., Identification Methods of Terpenoid Gum Bases Using TLC and GC/MS, J. Vol. 51, No.5, October 2010.
- Scalarone D, Duursma MC, Boon JJ, Chiantoire O. MALDI-TOF mass spectrometry on cellulosic surfaces of fresh and photo-aged di- and triterpenoid varnish resins. Mass. Spec. 2005, 40, 1527-1535. doi:10.1002/jms.893