Introduction
Hemp, or industrial hemp is one of the fastest growing plants. It has been grown mostly for the industrial uses of its derived products, such as fiber, clothing, paper, paint, insulation, food, and animal feed (1).
The legality of industrial hemp varies widely between countries. Some governments regulate the concentration of THC and permit only hemp that is grown with low THC content. Normally, industrial hemp contains below 0.3% THC, while kinds of hemp grown for medicinal or recreational use can contain anywhere from 2% to over 20% THC.
The use of cannabis as medicine suggests that cannabis can reduce nausea and vomiting during chemotherapy, improve appetite in people with HIV/AIDS, and reduce chronic pain and muscle spasms. Medical cannabis can be administered through various methods, including capsules, lozenges, tinctures, dermal patches, oral or dermal sprays, cannabis edibles, and vaporizing or smoking dried buds.
As more countries legalize cannabis, increasingly stringent testing requirements are being developed on the state level for pesticide levels in cannabis products, since the pesticides are potentially toxic to humans and can lead to acute and chronic health effects due to bioaccumulation. Thus, a growing need for pesticide analysis, and the list of pesticides regulated throughout the world continue to increase.
The QuEChERS method is a widely accepted method to extract pesticides from food matrices; however, it has not proven to be suitable for pesticides extraction from cannabis. Pesticide analysis of cannabis is a challenge due to the large number of pesticides to monitor, the low method detection limits, and difficult cannabis matrix. In particular, dramatic difference in concentrations between pesticide traces and co-extracted matrix components such as cannabinoids and terpenes lead to severe matrix effects described later while high matrix load onto the GC-MS system results in overall system contamination, degradation of performance, frequent interruption for user maintenance.
There is a need for a simple and efficient method for the extraction of pesticides from cannabis that yields high recoveries and repeatable results. This work we propose the QuEChERS method complemented with additional SPE clean-up step. Resulting extract is then analyzed for residual amount of pesticides by gas chromatograph with mass-spectrometric detector manufactured by Q-Tek (Montenegro).
Experimental
Standards and Reagents
All reagents used in this work were HPLC grade. Pesticide standard mixes were commercially obtained from Restek (USA) (Restek, Cat.# 32568; 32567; 32565; 32564; 32567; 32277). 4-Chlorodiphenyl ether (Restek cat# 31620) was used as internal standard.
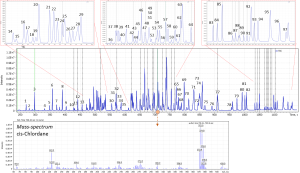
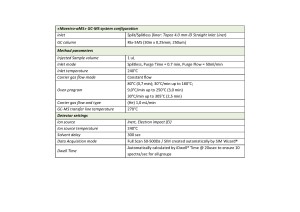
The extracted samples were analyzed on Q-Tek single quadrupole GC-MS. The GC-MS method parameters are outlined in Table 1. TIC of the standard mix containing 98 pesticides at a given separation conditions is shown on fig.1
Fig 1. TIC of 98 pesticide standards mix.
1-Methane, bis(2-chloroethoxy)-; 2-1,3,5-Trichlorobenzebe; 3-Dichlorvos; 4-1,2,4,5-Tetrachlorobenzebe; 5-Naphthalene, 6-2-chloro-; 7-Mevinphos; 8-Etridiazole; 9-Cloroneb; 10-Benzene, pentachloro-; 11-Benzene, 1-chloro-4-phenoxy-; 12-Demeton-O; 13-Ethoprophos; 14-Naled; 15-Phorate; 16-Benzene, 1-bromo-4-phenoxy- (ISTD); 17-HCH isomer I; 18-Demeton-S; 19-Benzene, hexachloro- (HCB); 20-2,3,4,5,6-pentachloro anisole; 21-Atrazine; 22-HCH isomer II; 23-Terbuthylazine; 24-HCH isomer III; 25-Diazinone; 26-Pyrimethanil; 27-Tefluthrin; 28-Disulfoton; 29-Terbacil; 30-HCH isomer IV; 31-Endosulfan ether; 32-Transfluthrin; 33-Vinclozoline; 34-Parathion-methyl; 35-Heptachlor; 36-Fenchlorphos; 37-Pentachlorothioanisole; 38-Fenthione; 39-9,10-Anthracenedione; 40-Triadimefon; 41-4,4-Dichlorobenzophenone; 42-Trichloronate; 43-Cyprodinil; 44-MGK 264; 45-Isodrine; 46-Penconazole; 47-Fipronil; 48-Triadimenol; 49-Captan; 50-Triflumizole; 51-Procymidone; 52-Folpet; 53-Chlorbenside; 54-trans-Chlordane; 55-Paclobutrazol; 56-Tetrachlorvinphos; 57-Endosulfan; 58-Flutriafol; 59-Ovex; 60-Prothiofos; 61-Fludioxonil; 62-p,p’-DDE; 63-Myclobutanil; 64-Flusilazole; 65-Chlorfenapyr; 66-Fensulfothion; 67-beta-Endosulfan; 68-p,p’-DDD; 69-trans-Nonachlor; 70-Endrin aldehyde; 71-Clordecone; 72-Endosulfan sulfate; 73-o,p’-Methoxychlor; 74-Hexazinone; 75-Tebuconazole; 76-Captafol; 77-Iprodione; 78-Bifenthrine; 79-Phenothrine; 80-Pyriproxyfen; 81-lambda-Cyhalothrin; 82-Acrinathrin; 83-Fenarimol; 84-Permethrin isomer I; 85-Permethrin isomer II; 86-Coumaphos; 87-Cyfluthrine isomer I; 88-Cyfluthrine isomer II; 89-Cyfluthrine isomer III; 90-Cyfluthrine isomer IV; 91-Cypermethrine isomer I; 92-Cypermethrine isomer II; 93-Flucythrinate isomer I; 94-Etofenprox; 95-Flucythrinate isomer II; 96-Fluridone; 97-Fenvalerate; 98-Fluvalinate.
Table. 1. GC-MSD method parameters.
Q-Tek proprietary data processing algorithm SIM Wizard ® was used to automatically build SIM method based on full SCAN data from pure standard mix containing all analyzed pesticides at 1 ppm each. The SIM-library was, then, used to facilitate user’s choice of most informative and optimal ions to be monitored during SIM acquisition. To maximize the system sensitivity iDwell®Time algorithm was engaged. iDwell®Time algorithm automatically groups the target compounds into time segments so, that dwell time on each of the monitored ion mass is as long as needed (see fig.2) The both proprietary algorithms described above are nice productivity tools that help to quickly, within a minute, optimize the data acquisition parameters of any new SIM-method and to obtain high intensity signal, ideal peak shape and to reach statistics on all target masses including those for the analytes present at their trace concentrations in the sample.
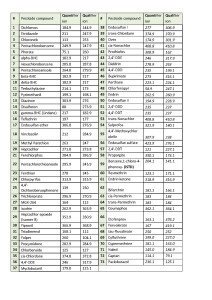
The list of monitored ions selected for all the target compounds are shown in Table 2.
Fig.2 TIC chromatogram automatically split into 22 time segments, each segment to include the least number of monitored ions for highly sensitive sample scanning in SIM mode for all target 73 analytes and the ISTD. The process is fully automated and takes about a minute from TIC chromatogram integration to getting an optimized SIM method.
Table 2. List of ions to be monitored during data acquisition in SIM mode.
Sample preparation
Industrial hemp extracts were used in this study. The matrix composition is very complex and contains compounds at various concentration levels from different classes such as cannabinoids, terpenes, hydrocarbons, sugars, fatty acids, flavonoids, and others. Hemp leaf and inflorescense may contain upto 60 cannabinoids, typically present in acidic forms and having carboxyl group in position 2 of the phenolic part of the molecule. Following hemp plant growth and ripening the cannabidiols and tetrahydrocannabinols are predominantly present in the hemp plant while in result of plant aging or in result of processing the THC is transforming into cannabinol. Therefore, there is a wide variety of co-extracted matrix components whose level depends on hemp plant cultivation conditions, on particular parts of plant sampled, or on the kind of hemp derivative product being analyzed. Such cannabis matrix complexity poses specific challenge on extraction of pesticide traces and later on leads to ion suppression and matrix interference during MS-experiments. Therefore, part of the present work was devoted to development of efficient clean-up of hemp matrix extract that would allow analyst to reliably detect and quantify target analytes in resulting sample injected into GC-MS.
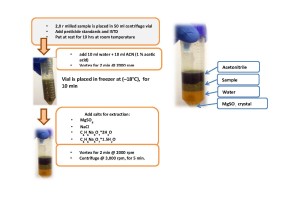
The starting point of sample preparation improvement was QuEChERS method well described elsewhere (2). The use of QuEChERS method, initially, involved ACN extraction of target compounds from dry hemp sample followed by matrix clean-up with salts and ion-exchange sorbent Bondesil-PSA at presence of graphitized carbon black (GCB), as shown on fig. 3 and 4.
Fig. 3. Hemp sample extraction by QuEChERS method, Stage 1.
Fig. 4. Matrix clean-up with salts and ion-exchange sorbent Bondesil-PSA at presence of graphitized carbon black (GCB) by QuEChERS method.
However, QuEChERS method alone has proven to be insufficient to produce the extract clean enough to be analyzed on single quadrupole GC-MS. Even the extract resulting from PSA clean up step was found to contain number of interfering matrix components at high concentrations (fig. 6).Therefore, it was decided to add one more sample prep step as further described herein (fig. 5).
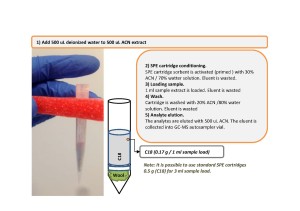
For this additional sample clean-up step a SPE cartridge filled with C18 sorbent was used. The sample to load onto the SPE cartridge had to be diluted, first, so that 500uL of deionized water was added to 500uL of hemp extract. The cartridge was pre-conditioned prior to sample load, and subsequent sample clean-up was carried out as shown on fig.5.
Fig. 5. Additional sample preparation step following QuEChERS extraction. Stage 2: SPE Matrix clean-up
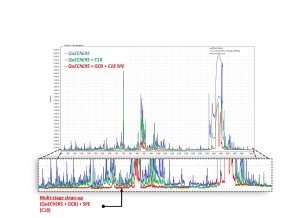
Results of three different sample preparation procedures in comparison are given in fig.6, showing Total Ion Chromatograms from three respective samples. Signal from matrix components decreased substantially after SPE clean-up. Therefore, such sample preparation method involving SPE clean-up of the extract was accepted as optimal, for the time being, and was used further in this work as so-called “clean matrix extracts” for matrix calibration. These clean extracts were spiked with calibration mix containing 73 pesticides at 20; 50; 100; 200 ppb in order to build matrix calibrations.
Fig. 6. Results of three different sample preparation procedures in comparison. Note sample prep procedures and respective level of chemical background noise from matrix components on TIC (blanks) are designated with the same colors.
Results and discussion
Linearity
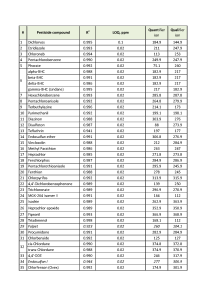
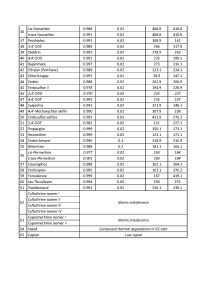
In order to evaluate ability of the GC-MS method to quantitate accurately target pesticides as well as to calculate respective LOQs, the matrix calibration was used for each compound having 4-chlorodiphenyl ether at 0.1ppm as Internal Standard. The LOQs were calculated as per EU Guidance (3) and resulting values were put together in Table 3.
Table 3. List of ions to be monitored, LOQs and linearity of calibrations for pesticides in hemp extracts.
Discussion
Important to note that not all the compounds present in the pesticide mix were used in the calculations. The HPLC amenable pesticides were excluded from the target list, as they were proven to be reliably quantitated by HPLC-ESI-MS method. So it was decided to use remaining 65 pesticides for further GC-MS quantitative method evaluation (Table 3). Another problem to tackle while working on hemp samples is 5-fold dilution of the pesticide concentrations during hemp extraction with solvent (2g of hemp is extracted with 10mL of ACN), thus, requiring from a GC-MS instrument to measure concentrations 5 times below their actual level in the sample. This fact has an effect on overall method LOQ parameter.
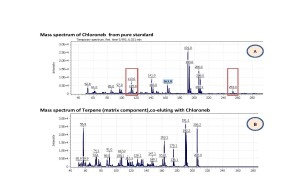
The results demonstrated, that 54 of total 65 pesticides could be confidently detected and quantified at 0.02ppm (0.1 ppm in dry hemp) or below. The rest 15% of pesticides either were possible to detect and quantify at higher concentration levels > 0.1ppm or were difficult or impossible to confidently detect due to severe matrix interferences enhancing or suppressing analyte signals on mass-chromatogram. As an example (fig. 7) pesticide Chloroneb is eluting closely with matrix component Terpene, both having mass-spectra so similar, thus making it nearly impossible for an analyst to choose the most abundant and widely referred to in literature ions for Chloroneb quantification.
Fig. 7. Similarity of mass-spectra from target analyte Chloroneb(A) and major matrix component Terpene(B) interfering with the analyte, thus, making it difficult to choose characteristic ions for this analyte while setting up SIM acquisition parameters.
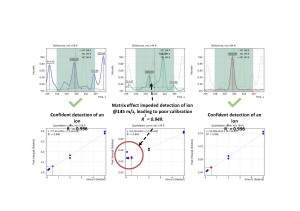
Another example is presented on fig.8 showing matrix overlap on α-BHC target ion at m/z =219. Only thorough and detailed look into α-BHC mass-spectrum would allow an analyst to choose an appropriate less abundant ion for confident detection of this compound, having it’s compromised method detection limit accepted as a trade-off.
Fig. 8. Matrix overlap on α-BHC target ion at m/z =219.
Besides, compromised detection limits for some pesticides are a result of the pesticide compound degradation at elevated temperature. Unstable Naled pesticide is known to degrade even at room temperature forming dychlophos, and therefore can only be detected together with dychlophos by peak of the latter.
Post-run data processing algorithm «Flexible SIM»
Quantitative analyses of complex plant extracts by single quadrupole GC-MS typically require meticulous work to individually select characteristic ions to be monitored for each target analyte during data acquisition in SIM mode. As discussed above, the matrix background may potentially overlap analyte signals acquired in SIM mode as shown on fig. 7-9 due to insufficient selectivity of single quadrupole mass-spectrometric detector in some difficult cases. In order to facilitate SIM method development task the novel algorithm “Flexible SIM” has been developed and implemented by Q-Tek.
Fig. 9. Matrix THC interference with analyte signal. Examples are Endrin ketone and Bifenthrin
Based on experimental data that had been acquired in SIM mode the “Flexible SIM” algorithm made it possible for an analyst to review and select a characteristic compound ion, which is the least affected by matrix, for quantitative analysis for each target analyte.
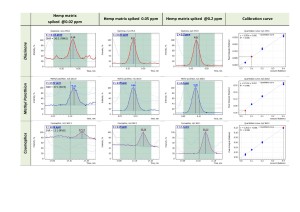
In particular, the “Flexible SIM” algorithm automatically builts calibration curves for all the ions listed in the data acquisition method. So for each target compound the analyst is given a possibility to toggle between the calibration curves and choose the one that meets quality criteria the best way for a given sample matrix (fig.10). Fig. 11 shows matrix calibration curves of a good quality for Diazinon, Methyl Parathion and Coumaphos obtained with help of “Flexible SIM” algorithm.
Fig. 10. «Flexible SIM» algorithm simultaneously builds calibration curves for all the ions that have been selected by the operator for a specific analyte, regardlessly of their role – be it a quantifier or a qualifier. Example is Dychlophos (@ 0.05 ppm).
Fig. 11. Matrix calibration for Diazinon, Methyl Parathion and Coumaphos obtained with help of “Flexible SIM” algorithm.
Conclusion
The GC-MS demonstrated ability to confidently detect pesticide residues in hemp extracts at their concentration level below 0.02 ppm
Novel data processing algorithm Flexible SIM much shortens time spent on selection of interference-free characteristic ions to be used for SIM method, while enables an analyst to reach detection limits mandated by various regulators for very complex hemp samples.
Two stage sample preparation procedure including QuEChERS method followed by SPE clean-up of the extract provided best result for purpose of this study. Still, a few pesticide compounds, namely Cyfluthrine, Cypermethrine, Endrin ketone, Bifenthrin were difficult to detect below 500 ppm level. We believe, more development work is required on sample preparation side to reduce interference of matrix component THC with their detection.
This study will be continued in direction of further improvement on selective acquisition modes for complex extracts with high matrix load.
References
- Pierre Bouloc. Hemp: Industrial Production and Uses CABI; 2013 edition (October 21, 2013).
- Anastassiades M., Lehotay S.J, Stajnbaher D, and F.J. Schenck //The Journal of AOAC International 86. P. 412–431(2003).
- Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food, 2016