Abstract
The capabilities of chromatography-mass spectrometry system for quantification of parabens in cosmetic products and hygiene products were demonstrated, shower gel and toothpaste were used as an example.
Introduction
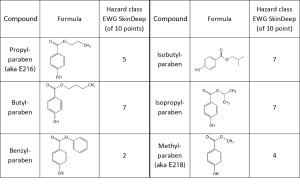
Parabens are esters of para-hydroxybenzoic acid, which due to their bactericidal and fungicidal action are most often used as preservatives for the production of a wide range of medicines and products for hygiene, cosmetics, food and children’s products. The most common ones in production are butylparaben, methylparaben and propylparaben.
According to the US Food and Drug Administration, the use of parabens in the production of cosmetics in the world has recently increased by 1.7 times compared to 1981. Today, parabens can be found in about 80% of personal care products. [1]
Despite the fact that parabens are still considered safe, various independent studies have obtained data on their ability to disrupt the function of the endocrine system when exposed to high doses [2]. However, the causal relationship between the use of parabens and their possible carcinogenic properties remains controversial [3, 4]. Parabens are known to cause allergic reactions, including skin irritation and contact dermatitis on sensitive and damaged skin [5]. The levels of parabens in samples of human biological materials depend on the frequency of use of consumer and cosmetic products with their content.
Parabens have estrogenic activity, that is, they act like a hormone. Moreover, this action increases with the length of the organic radical or chain. In particular, butylparaben has higher estrogenic activity compared to propylparaben, which is presented in Table 1 [6].
Table. 1. General information about the varieties of parabens.
As previously noted, there is no direct evidence that parabens pose or do not pose a danger to humans. However, the U.S. Department of health still set a maximum daily intake of parabens: 142.08 mg and 3.024 mg / kg (ppm) for adults and 37.8 mg for children (approximately 2.368 mg/kg (ppm) body weight) [7].
In the European Union, the permissible content of parabens in cosmetic products is 0.4% for certain types of parabens and 0.8% for mixtures of all parabens [8]. In 2011, the Danish government decided to impose restrictions on the use of some particularly dangerous parabens (propyl -, isopropyl -, butyl – and isobutyl) in personal care products, such as creams, intended for children under 3 years. Since 2014, France has banned the use of isopropyl-, isobutyl -, pentyl – and benzyl-parabens, as their effects on humans are little understood. But, despite this, there is no uniform legislation regulating the concentration of parabens in cosmetics in any of these countries [9].
Public organizations that promote awareness of cosmetic ingredients believe that further research is needed to determine the safety of parabens [12]. At the same time, public concern generated by data on the possible dangers of using parabens led consumers and companies to search for alternatives [13].
Experimental
Materials and methods
All reagents used in the experiment were classified as HPLC grade. Standards of target parabens had a purity of at least 99.5%.
Samples of toothpaste as well as a sample of shampoo purchased at a local supermarket were chosen as the subject of the study. The following concentration points were used to construct the calibration dependence: 1, 5, 10, 50, 100 ppb.
Table. 2. Parameters of the instrumental method.
| Q-Tek GC-MS system configuration | |
| GC-MS system | Q-Tek GC-MS |
| inlet | Split/Splitless (liner: Restek 4 mm x 6,3 x 78,5) |
| GC column | Rtx-5MS (30m x 0,25mm; 250um) |
| GC Method parameters | |
| Injected Sample volume | 1 uL |
| Inlet mode | Splitless; Purge Time = 1.2 min, Purge Flow = 75 ml/min |
| Inlet temperature | 250°C |
| Carrier gas flow mode | Constant Flow |
| Oven program | 75°C (1,2 min);
12,0°C/min up to 220°C, hold 2,0 min; |
| Carrier gas flow and type | (He) 1,0 mL/min |
| GC-MS transfer line temperature | 270°C |
| Detector settings | |
| Ion source | Inert, Electron impact (EI) |
| Ion source temperature | 230°C |
| Solvent delay | 4,0 min |
| Data Acquisition mode | SIM (see below details) |
| Dwell Time | Automatically calculated by iDwell® Time |
Sample preparation
Fig. 1. Extraction of a toothpaste sample.
1 gram of the analyzed sample (toothpaste or shampoo) is placed in a 10 ml volumetric flask, then 4 ml of 5% methanol solution in water is added and stirred on a magnetic stirrer for 10 minutes (Fig. 1). After that, the extract is filtered through a filter with a pore size of 3-5 microns. Next, the solid-phase extraction procedure is carried out on LiChrolut®RP-18 cartridges ((40-63 µm) 500 mg 6ml standard PP-tubes), according to the scheme below.
Scheme of solid-phase extraction
- Washing of the stationary phase – 4 ml of methanol.
- Conditioning phase- 4 ml of 5% methanol/95% water (*).
*An important condition for the successful completion of the stage is that the phase in the cartridge should not dry after this stage of conditioning.
- Sample loading – 4 ml of filtered sample extract.
- Washing the stationary phase – 4 ml of solution (5% methanol / 95% water).
- Drying the cartridge phase under vacuum.
- Elution – the final stage of target compounds elution is carried out with 1.5 ml of 100% methanol.
The resulting aliquot is centrifuged (Eppendorf, centrifuge 5415) at a speed of 14000 rpm, for 6 minutes. The supernatant extract layer is analyzed on Q-Tek GC-MS system. The list of target compounds and their detection parameters are presented in Table 3.
Table 3. The analytes’ peak Retention Time and the parameters of their scanning.
| № | Analytes | Quantifier ion | Qualifier ion
(Qual-1) |
Qualifier ion
(Qual-2) |
RT, min |
| 1 | Methylparaben | 121,1 | 93,0 | 151,9 | 8,39 |
| 2 | Ethylparaben | 121,1 | 137,9 | 165,9 | 8,97 |
| 3 | Propylparaben | 121,1 | 137,9 | 179,9 | 9,77 |
| 4 | Butylparaben | 137,9 | 121,1 | 194,0 | 10,55 |
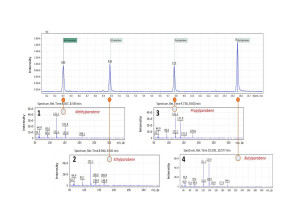
Fig. 2. Total Ions Chromatogram (SCAN) of paraben standards mix, concentration 5 ppm.
1 – Methylparaben; 2 – Ethylparaben; 3 – Propylparaben; 4 – Butylparaben.
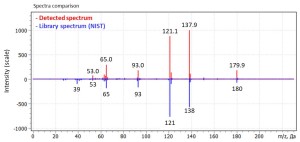
Fig. 3. Match of pure analyte spectrum acquired in Full Scan mode with the library spectrum (library NIST17, ver. 2,3)
Results and discussion
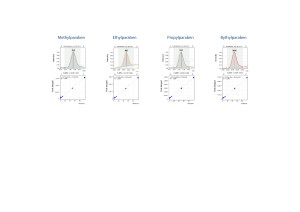
Quantification of parabens was carried out using external calibration. 5 calibration levels were used to build the calibration curve. The correlation coefficients for all target parabens were R2 ≥ 0.998 (Fig. 4).
Fig. 4. Linear calibrations and signals of the main ion currents for the target compounds.
Practical assessment of the method performance
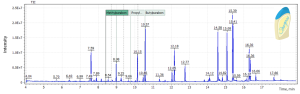
This method has been tested on various cosmetic samples, in particular toothpaste (2 samples) and shower gel. Figure 5 shows a toothpaste chromatogram (sample #1) where 3 parabens are detected.
Fig. 5. SIM chromatogram of toothpaste sample (#1).
Table. 4. The results of the analysis of the toothpaste sample (#1).
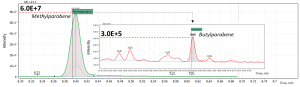
It is worth noting that the mass spectrometric detector allows you to detect target signals in wide dynamic ranges of concentration. In particular, in the toothpaste sample (#1), detection of methylparaben at the level of 2290 ppb and butylparaben at the level of approximately 0.2 ppb can be simultaneously observed. The difference in their detected signals was 10,000 times, which takes 4 orders of magnitude of the dynamic range of the system while analyzing high matrix samples.
Fig. 6. Detection of analytes at different concentration levels in a toothpaste sample (#1).
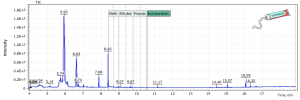
Figures 7 and 8 also show chromatograms of shower gel and toothpaste samples (sample #2). In the shower gel sample Ethylparaben was detected at 2.0 ppb, as well as high concentration of butylparaben at 1140 ppb (1.14 ng / µl). In the toothpaste sample (sample #2), methylparaben was detected at a concentration of 229 pb (0.23 ng/µl) and trace amounts of Ethylparaben at 5.6 ppb.
Fig. 7. TIC chromatogram (SIM) of the shower gel sample.
Table. 5. Results of analysis of shower gel and toothpaste samples (#2).
| Methylparaben | Ethylparaben | Propylparaben | Butylparaben |
| Target analyte concentrations in shower gel sample (#2) | |||
| Not detected | 2,02 ppb | Not detected | 1140,0 ppb |
| Target analyte concentrations in toothpaste sample (#2) | |||
| 229,0 ppb | 5,65 ppb | Not detected | Not detected |
Fig. 8. TIC (SIM) chromatogram of toothpaste sample (#2).
Summary and Conclusion
The experimental results prove the single quadrupole GC-MS system (Q-Tek D.O.O, Montenegro) can be effectively used for confident quantification of the target ingredients such as parabens in cosmetic products for various quality control or research purposes.
The single quadrupole Q-Tek GC-MS system allows to detect esters of para-hydroxybenzoic acid in a wide range of concentrations. This makes it possible to reliably control not only the presence of parabens in samples, but also to determine their quantities at trace levels against the background of high-intensity matrix components and other parabens.
References
- Pouillot A. J. Mеd Esthеt Chir Dermatol 2006; v. 33, p. 187-90.
- Harvey P. W., Everett D. J. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumors // Journal of Applied Toxicology journal. – 2004. – January (vol. 24, no. 1). – P. 1 – 4. – DOI: 10.1002/jat.957. – PMID 14745840.
- Monica Giulivo et al. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review
- Golden R., Gandy J., Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health // Critical Reviews in Toxicology: journal. — 2005. — Vol. 35, no. 5. — P. 435—458. — DOI: 10.1080/10408440490920104. — PMID 16097138.
- Kirchhof, M.G. & de Gannes, G.C. (2013) The health controversies of parabens. Skin Therapy Letter, 18(2), 5-7. PubMed PMID: 23508773.
- Cashman A.L, Parabens: a review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis, v.16 (2), p. 57–66
- CIR, 2008
- Официальный журнал Европейского Союза, 2009
- Kirchhof M.G, Skin Therapy Lett 2013; 18, 5–7
- ТР ТС — 009 / 2011
- The truth about antiperspirants and breast cancer. Unilever.
- Rita Arditti. Cosmetics, parabens, and breast cancer. Organic Consumers Association.
- Lebovits S.C. Cosmetic’s firms heed calls for organics. The Boston Globe.