Koluntaev Dm. Ph.D in biology,
Q-Tek d.o.o., Bar, Montenegro
Summary
The advantages and capabilities of the chromato-mass spectrometry system with an inert ceramic electronic ionization source are demonstrated by analyzing the profiles of human urine extract.
Introduction
The reliability of mass spectrometric detection of a compound directly depends on the sensitivity of the system to this compound. Sensitivity, in turn, is a result of the signal intensity, which depends on the initial number of ions of this compound formed in the ion source. Thus, a mass spectrometric detector may exhibit certain increased specificity for some groups of organic compounds vs the others.
Various organic compounds are known to have different ionization cross-section, based on atoms and functional groups that form its molecule [1]. Therefore, during ionization, each of the compounds will form a different, specific number of ions.
The most commonly used source of electron ionization in gas chromato-mass spectrometry is the so-called “closed type”. It further concentrates the ions of the compounds during ionization process and, thus, forms the maximum ion output to achieve higher sensitivity relative to the background gas. Not only the physical characteristics of compounds can play a role in the sensitivity and specificity of the analytical method. In many ways, the design of the ion source also plays a significant role, in particular, the surface quality of machined parts, magnetic properties of steel, sorption properties and chemical purity of inner surfaces.
This article presents a comparative analysis of two electronic ionization sources: a conventional type source (made of stainless steel), and an inert ceramic ion source designed specifically for samples of biological origin. An inert ceramic ion source is a standard option for chromato-mass spectrometric detector and is recommended for use in routine laboratories.
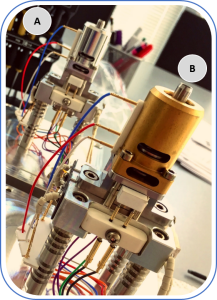
Fig. 1. Electron impact ion sources: А – conventional stainless-steel ion source; B – inert ceramic novel ion source.
Experimental
Materials and methods
The experimental part of the study involved two stages. At the first stage, we used a standard mix of 75 pesticides with 20 semi-volatile organic compounds as a test sample. Detector response to these compounds of the mix was recorded. At the second stage, we studied the efficiency and possible advantages of a ceramic source when working on biological objects. As the object of the study, human urine extracts were selected, that had been tested positive for presence of trace amounts of drugs of abuse.
Urine extracts were prepared and provided for comparative analysis by forensic toxicology laboratories. It is also worth noting that the choice of the research object was due to the fact that clinical samples, such as urine, blood, and body tissues, may contain a large number of co-extractive compounds that can adversely impact stability of MS detector signals due to various effects in ion source operation.
All reagents used for the experiment were classified as highly pure or HPLC grade. Standards for active pesticide substances, as well as a mixture of organic semi-volatile compounds, were at least 97% pure.
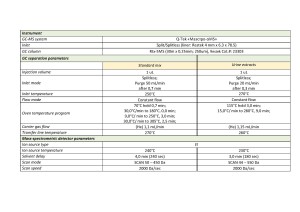
Table 1. Data acquisition method parameters.
Results and discussion
The first part of the experiment.
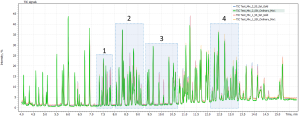
Figure 2 shows an overlay of chromatograms from four consecutive runs of the standard mix in the Q-Tek Analyst (SOFTWARE package):
– 2 injections are made on a standard stainless-steel source.
– 2 injections are performed on an inert ceramic source.
In figure 2.1 below the selected sections of the chromatogram are zoomed in for more detailed view. As one can see in signal profiles, there is a significant difference in the response for some of the compounds. Then, by means of “auto integration” algorithm peaks on the profiles were integrated, the compounds identified against library.
Fig. 2. Overlay of four TIC chromatograms from a mixture of standards.
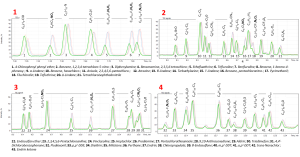
Fig. 2.1. Zoom-in view of selected sections of the chromatogram.
The Q-Tek Analyst auto-integration algorithm processes data acquired in full scan mode. In particular, the process involves automatic detection of chromatographic peaks on each specific ion chromatogram for each ion mass of the range. Further, the detected peaks are grouped by their retention times, forming isolated spectra of test sample components cleared from the chemical background. In the final step of the algorithm, an automatic search in user selected mass spectrum libraries is performed to find the best match and propose a compound name for each peak. The results of auto-integration are shown in figure 2.1.
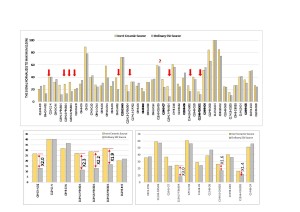
According to the data obtained, the difference in signal responses is observed in compounds that contain both nitrogen and oxygen atoms together (hereinafter “NO-compounds”). At the same time, in figure 3, it is possible to estimate the differences as two-fold increase in the signal intensity on the inert ceramic source compared to the signal from the same compounds acquired on a standard stainless-steel source.
Fig. 3. Diagram of the relative signal response to compound from standard mix for two types of ion sources in comparison. Zoom-in panes for “NO-compounds” charts.
For other groups of organic compounds, this effect was not observed. It is also worth noting that the exception was the signal of the vinclozoline compound, which had a similar intensity at both sources. A detailed analysis of the normalized signals to the maximum signal in the profile is presented in table 2.
Table 2. A detailed analysis of the signals of standard substances.
The second part of the experiment.
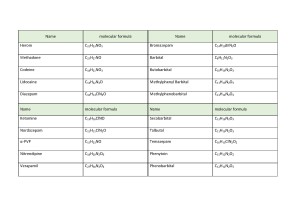
To prove the initial observation and see its potential practical implications, further experiments were run on urine extracts to check for relative intensity of NO-compounds from the “real world” challenging matrix. Number of drugs are NO-compounds and might be expected to produce elevated detector response on inert ceramic source. Table 3 shows some of those medicinal and narcotic drugs, as an example.
Table 3. List of some medicinal and narcotic drugs NO-compounds.
Comparison of signals of target compounds was calculated relative to other compounds present in the urine profile but differing in atomic composition. The results of studies of three different urine extracts are shown in figures 4a – 4c.
The obtained results show the detector signals from nitro compounds (“NO-compounds”) to increase nearly two-fold when using a ceramic electron impact ionization source compared to those from a stainless steel source.
Thinking further on practical advantages, the inert ceramic ion source can be used not only for more efficient screening of biological fluids for drugs of abuse, but also for the detection of explosive traces, as 2,4-dinitrotoluene (C7H6N2O4), trinitrotoluene (C7H5N3O6), octogen (C4H8N8O8), hexogen (C3H6N6O6), trinitrobenzene (C6H3N3O6) can also be so-called NO-compounds. Many of explosives have both nitrogen and oxygen atoms in their structure, which implies increased sensitivity and reliability of their mass spectrometric detection on with inert ceramic ion source.
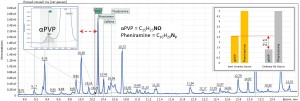
Fig. 4a. The result of a study of human urine, comparing the signals of the α-PVP component (C15H23NO) and the amitriptyline signal (C20H23N), detected also in the profile.
Fig. 4b. The result of a study of human urine, comparing the signals of the α-PVP component (C15H23NO) and the phenyramine signal (C16H20N) present in the urine profile.
Fig. 4c. The result of a study of human urine, comparing the signals of the component cotinine (C10H12N2O) and dioctyl phthalate (C24H38O), also present in the profile.
Conclusions.
- The use of an inert ceramic source of electronic ionization on Q-Tek allows increasing the sensitivity of the analytical method when detecting nitrogen-containing compounds.
- The practical application of the ceramic source covers a wide range of target nitrogen-containing compounds in laboratory practice, in particular pharmaceuticals and narcotic compounds, target substances in the field of ecology (pesticides), as well as most compounds from the class of explosives.
Literature
- Karl K. Irikura, Partial Ionization Cross Sections of Organic Molecules, , Journal of research of the National Institute of Standards and Technology, Volume 122, Article No. 28 (2017).