Introduction
Following advances of new analytical methods to determine low concentrations of target analytes, the interest in research of various biological matrices that differ from previously widely used ones (blood, urine) has also grown significantly in clinical practice. The most visible example is the study of human saliva. This is confirmed by the growth of scientific articles and publications over the past few years, which note the use of saliva as an easily accessible human biological fluid [1 – 5].
The advantages of analyzing saliva include a painless and non-damaging method of collecting clinical samples, which is very important for people, for example, who suffer from hemophilia or are afraid of venipuncture. In addition, the reaction in response to venipuncture can affect the level of glucocorticoids, which should be taken into account when diagnosing Itsenko-Cushing’s disease. Daily collection of saliva to determine the phase of the menstrual cycle, circadian rhythm, pharmacokinetics of medicines is possible at home, as well as during sports competitions, leading to recognition of the convenience of using saliva as a biological matrix for analysis [5].
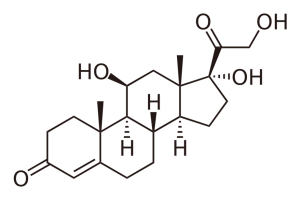
The interest of clinicians in study of saliva is also fueled by its potential value for the diagnosis of systemic and local pathological state. Numerous observations demonstrate the relationship of diseases of the salivary glands, as well as the composition of saliva with diseases of other organs and their systems, such as lesions of the adrenal and pancreas, acute and chronic respiratory diseases, diabetes, etc. [6, 7]. It is noted that the salivary glands are actively involved in maintaining General hormonal homeostasis due to their intrinsic intersecretory function [6, 8, 9]. Therefore, attempts have long been made to determine steroid hormones in saliva (table 1), which are believed to represent a free fraction of blood steroid hormones [10, 11].
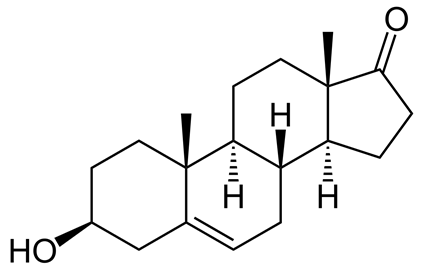
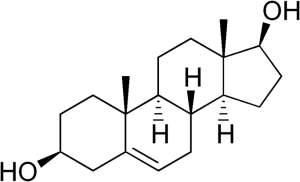
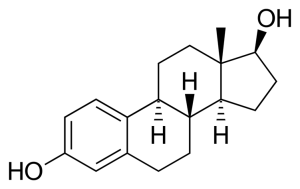
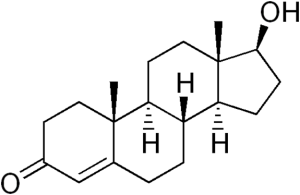
Table 1. List of steroid hormones, their characteristics and structural formulas.
Day to day a person is exposed to numerous stressors, a body in its response releasing catecholamines and steroid hormones. Determination of catecholamines in saliva, due to their instability and low concentration, is nearly impossible. Therefore, in order to assess an extent of chronic stress the best is to analyze increase of cortisol level. Saliva sampling, in contrast to blood sampling, does not cause psychological stress in the subject. Therefore, a study of the level of cortisol in saliva for the psychological assessment of stress-unstable patients is more preferable. However, cortisol concentration in saliva is significantly lower than that in blood plasma, which often acts as a limiting factor in determining the correlation between the level of this hormone and the development of chronic stress [5].
Determination of cortisol in saliva is used not only to assess the stress states, but also to facilitate diagnosing such pathological processes as the phenomenon of hypercorticism (Itsenko – Cushing syndrome). Hypercorticism syndrome is manifested in excessive formation of adrenal cortex hormones, and most often endogenous hypercorticism is caused by increased production of adrenocorticotropic hormone in the pituitary gland. It can occur against the background of an overdose of glucocorticoids in the treatment of various diseases (exogenous hypercorticism). Quite often, cortisol hypersecretion is observed in obesity, chronic alcohol intoxication, pregnancy, and some mental and neurological diseases (functional hypercorticism). In patients with hypercorticism, cortisol secretion is increased around the clock. That is why round-the-clock monitoring of cortisol levels is more convenient to perform on saliva, rather than on blood plasma. An important point for these patients is that such a study does not require their hospitalization, and the collection of saliva can be carried out at home for subsequent analysis in the laboratory.
With congenital hyperplasia of the adrenal cortex (changes in the structure of tissues, accompanied by an increase in the number of cells that make up them), the cortisol precursor – 17-hydroxyprogesterone is quantitated at high concentrations in saliva three times a day. Thus, measured concentrations allow a clinician to evaluate effectiveness of replacement therapy for congenital adrenal hyperplasia with cortisol, prednisone and dexamethasone. In many forms of congenital hyperplasia of the adrenal cortex, the secretion of androgens in these endocrine glands increases. This leads to virilization of female patients. Therefore, in addition to the study of 17-hydroxyprogesterone in saliva, the level of Androstenedione can be determined.
The appearance of typical signs of puberty is preceded by an increase in the activity of the adrenal glands, which is reflected in the level of dehydroepiandrosterone (DHEA). DHEA is formed in the adrenal cortex and partially in the sex glands from 17-hydroxyprogesterone and through the phospholipid layer of epithelial cell membranes located next to blood vessels freely enters the oral cavity and saliva. At the same time, low levels of DHEA in saliva are detected when puberty is delayed, and its increase is observed in premature puberty. It is also worth noting that according to literature data, there is no correlation between DHEA levels in saliva samples and blood plasma. Also, the quantitative characteristic of DHEA is a marker of stress in psychological research and in sports medicine.
The determination of female and male sex hormones (androgens, estrogens, and progesterone) in saliva is of important clinical significance. According to the study of the concentration of estradiol and progesterone in saliva, the phases of the ovulation cycle are determined. Also, determining the concentration of progesterone is an important indicator for evaluating ovarian function. In particular, the concentration of progesterone in saliva in the follicular phase is significantly less, and in the preovulation period, its concentration reaches peak values. It is worth noting that the determination of progesterone in saliva (less often estradiol) must be carried out in conjunction with the detection of the level of these hormones in the urine. Many studies have shown that the concentration of testosterone in saliva correlates well with the concentration of the free fraction of testosterone in the body. The amount of testosterone in saliva is used to detect male hypogonadism or eugonadism.
Androstenedione is formed in the adrenal glands and gonads and is an intermediate in the synthesis of both testosterone and estrone. In women, the secretion and rate of Androstenedione formation are higher than those for testosterone, and the extra-adrenal conversion of Androstenedione to testosterone is significantly pronounced. The highest levels of Androstenedione are determined in the morning. Elevated levels indicate congenital virilizing adrenal hyperplasia, polycystic ovary syndrome, stromal ovarian hypertecosis, 3β-hydroxy-steroid dehydrogenase deficiency, and other cases of hirsutism (male-type hair loss) in women with virilizing tumors of the adrenal glands or the ovaries. In the saliva of women, the concentration of Androstenedione is normally low, and in hirsutism it increases by 2-6 times. In men, the level of this hormone in the saliva does not differ from that of women.
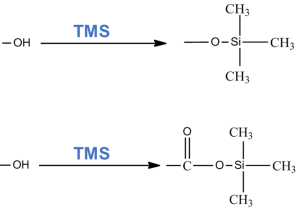
The purpose of this study is to develop a sensitive and multi-component method for determining steroid hormones in human saliva by means of gas chromatography-mass spectrometry, to get a fast and highly sensitive result. The selectivity of the method makes its application particularly advantageous when working with complex biological samples, where it is often required to exclude the strong influence of matrix interfering components of the sample. The process of silylation of target hormones is a universal method used to give them the properties of volatility of organic compounds, resulting in a sensitivity of GC-MS method.
Experimental part
Materials and methods
Methanol, isopentane, and bidistilled water – all reagents used in the experiment were classified as particularly pure or “HPLC grade”. As a derivatization procedure, a silylation reaction was used to form trimethylsilyl derivatives using the MSTFA derivatizing agent with ammonium iodide NH4I, as well as dithioerythritiol (DTE) as a catalyst (the ratio of components in the MSTFA/NH4I/DTE mixture is 100%/2%/1.5% (v/w/w)). The internal standard used was the standard methyl ester of testosterone.
To prepare a mixture of standard substances, separate standards for each steroid were used, with a purity of at least 99%. A mixture of standard solutions was prepared in methanol with a primary concentration of 1 mg / ml for each compound.
The procedure of saliva collection and extraction of steroids
Saliva samples obtained from an accredited laboratory of the Institute of Analytical Toxicology were used for trial and assessment of the proposed technique.
Methods of collecting and storing saliva affect the level of hormones in the saliva. There are various ways to get saliva. They include collecting the saliva without stimulation as well as using stimulants such as paraffin, citric acid, etc. However, when analyzing stimulated saliva, keep in mind the effect of dilution due to the high-water content compared to non-stimulated saliva. In this regard, samples were taken without additional stimulation using a moisture-absorbing cotton swab (Fig. 1) placed in the cheek space of the oral cavity (for 5 min).
 Fig. 1. Specialized cartridge for saliva extraction with a moisture-absorbing cotton swab (after centrifugation of the cartridge).
Fig. 1. Specialized cartridge for saliva extraction with a moisture-absorbing cotton swab (after centrifugation of the cartridge).
Sampling was carried out in the morning by specialists in accordance with the due procedure for saliva collection. In cases where immediate analysis was not possible, saliva samples were frozen or stored at a temperature of -5°C to -18°C.
A 1.0 ml saliva sample was placed in a 10 ml tube and a 20.0 ml solution of internal standard testosterone methyl ether (1.0 mcg/ml (ppm) in methanol) was added to the test sample. The solution was applied to a prepared conditioned cartridge for solid-phase extraction with a capacity of 1 ml and the water was disposed of under vacuum, while binding the target components to the polar phase of the sorbent. To elute the components from the sorbent, 1.0 ml of methanol was used, then the resulting organic phase was dried using an Eppendorf rotary evaporator (Concentrator 5301) for 40 minutes at 60°C.
Derivatization
50 µl of silylating reagent (MSTFA + 2.0% NH4I + 1.5% DTE, v/w/w) was added to the dried residue, then the vial was placed in a thermostat at 90°C for 10 min. 2.0 µl of resulting derivative was injected then into gas chromatograph.
Gas chromatography and mass spectrometry
Mass spectra and signals of structural characteristic ions were measured by Q-Tek GC-MS. The molecules were ionized in electron impact ionization source at 70 eV ionization energy and at a temperature of 230°C. Full-scan mass spectra (m/z 50-700) were obtained, first, in order to identify and study the structural characteristics of the target compound ions. Separation of the steroid mixture was performed on a fused silica capillary column.
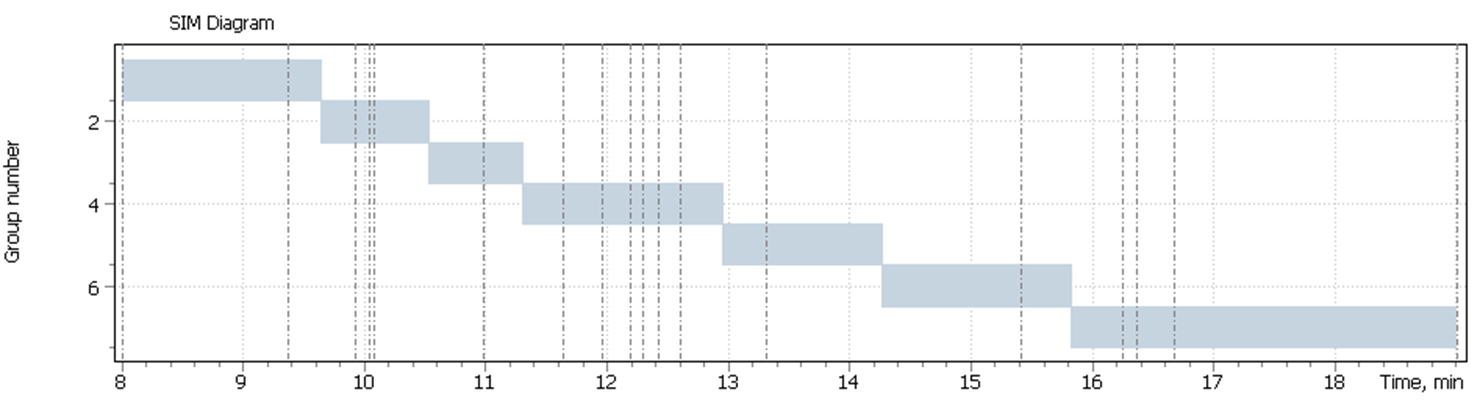
The parameters of the chromatographic separation and the mass detector data acquisition parameters are shown in table 2 and 3, respectively.
Table. 2. Instrumental method parameters.
Table. 3. Retention Time of the target ions peaks and their scanning parameters.
| № | List of steroid derivatives | Quant ion | Qualifier-1 | Qualifier-2 | RT, min |
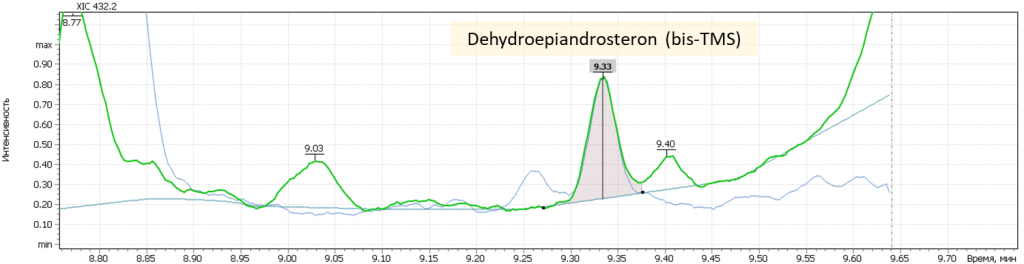
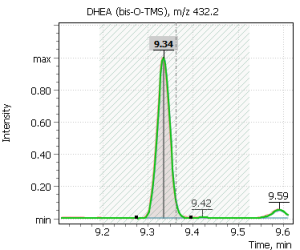
| 1 | DHEA (bis-O-TMS) | 432.2 | 417.2 | 434.2 | 9.36 |
| 2 | Androstenedion (bis-O-TMS) | 430.2 | 415.2 | 431.2 | 9.92 |
| 3 | Estradiol (17β-) (bis-O-TMS) | 416.2 | 285.1 | – | 10.04 |
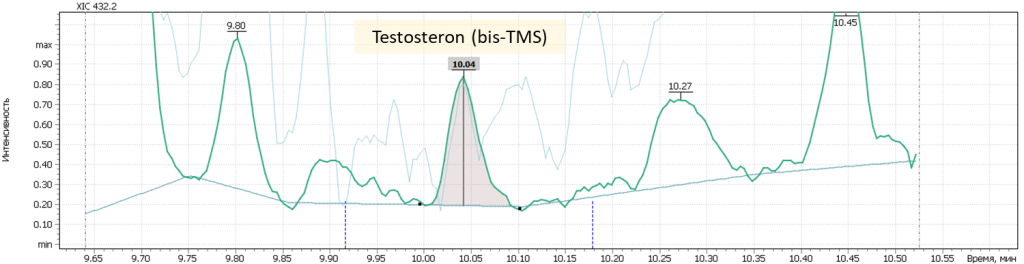
| 4 | Testosteron (bis-O-TMS) | 432.2 | 431.2 | – | 10.07 |
| 5 | methyl-ester-Testosteron (bis-O-TMS) ISTD | 446.3 | 301.2 | – | 10.98 |
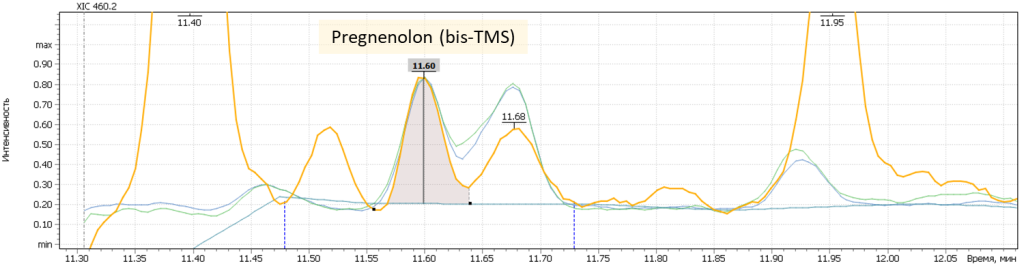
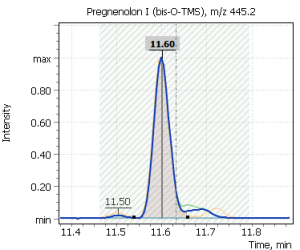
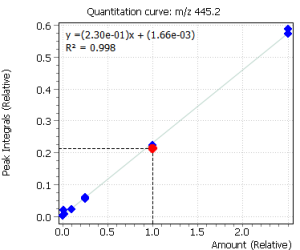
| 6 | Pregnenolon I (tris-O-TMS) | 445.2 | 460.2 | 446.2 | 11.63 |
| 7 | Pregnenolon II (tris-O-TMS) | 445.2 | 460.2 | 446.2 | 11.96 |
| 8 | Progesteron I (tris-O-TMS) | 458.2 | – | – | 12.19 |
| 9 | Progesteron II (tris-O-TMS) | 458.2 | 443.2 | 157.1 | 12.29 |
| 10 | 17α-Hydroxyprogesteron (mono-TMS) | 474.2 | 431.2 | 433.2 | 12.42 |
| 11 | Progesteron III (tris-O-TMS) | 443.2 | 458.2 | – | 12.60 |
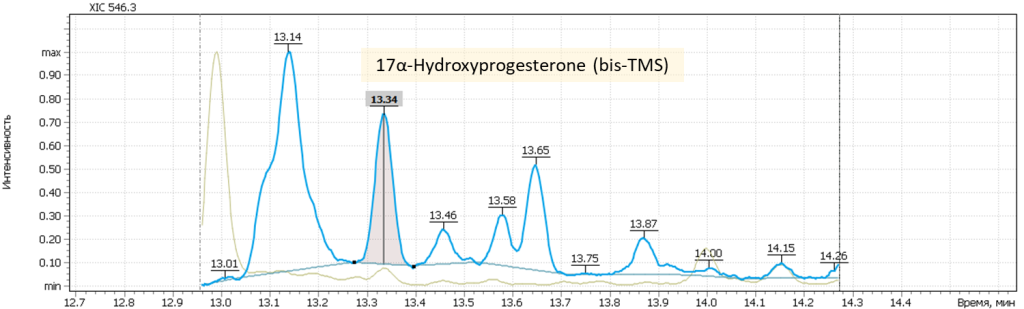
| 12 | 17α-Hydroxyprogesteron (bis-O-TMS) | 546.3 | 301.2 | 441.2 | 13.31 |
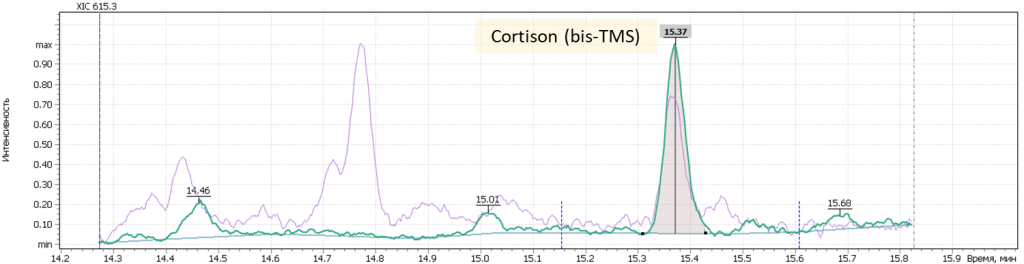
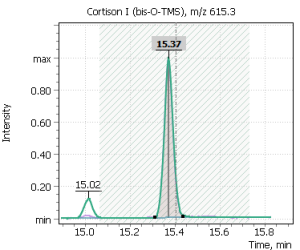
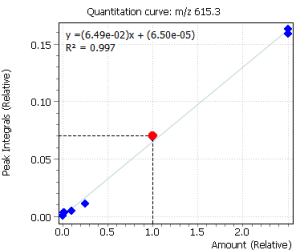
| 13 | Cortison (bis-O-TMS) | 615.3 | 630.3 | – | 15.4 |
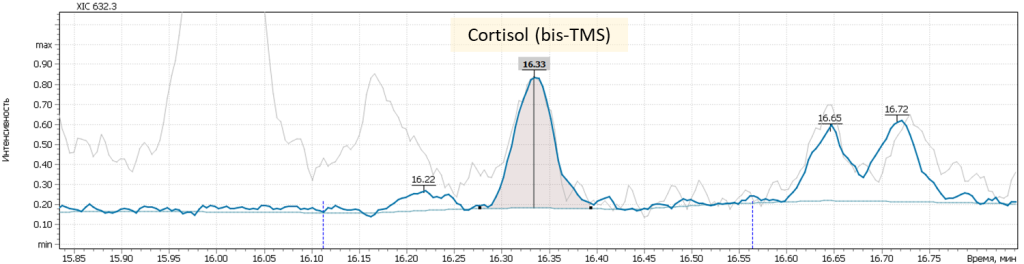
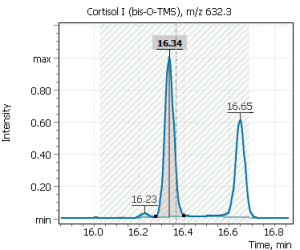
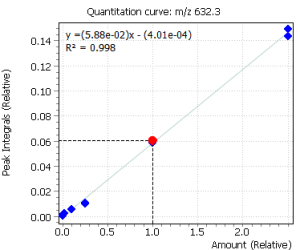
| 14 | Cortisol I (tetrakis-O-TMS) | 632.3 | 634.3 | – | 16.36 |
| 15 | Cortisol II (tetrakis-O-TMS) | 632.3 | 634.3 | – | 16.67 |
Linearity
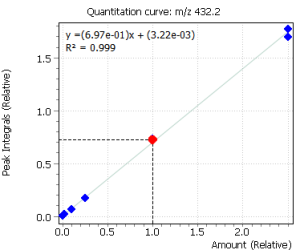
Calibration curves for all the analyzed compounds were obtained after their extraction with subsequent derivatization from 1.0 ml of bidistilled water. The concentration range for all compounds was from 0.05 ng / ml to 50 ng / ml of water (based on 7 calibration levels). Internal standard was used to quantify the compounds in saliva.
| 17α-Hydroxyprogesterone | Androstenedione | Dehydroepiandrosterone |
 |
 |
 |
 |
 |
 |
| Cortisol | Cortisone | Pregnenolone |
 |
 |
 |
 |
 |
 |
| Progesterone | Testosterone | Estradiol (17β-) |
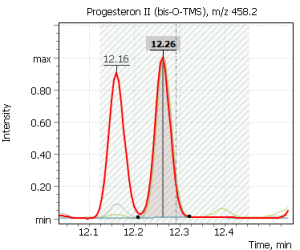 |
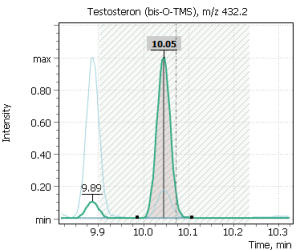 |
 |
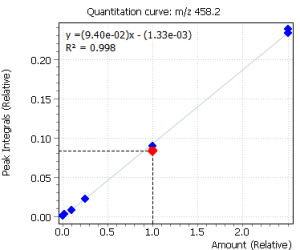 |
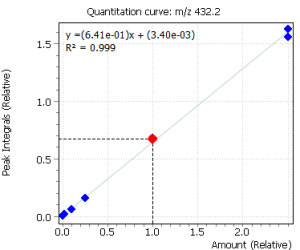 |
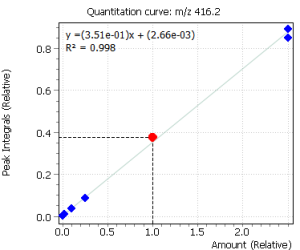 |
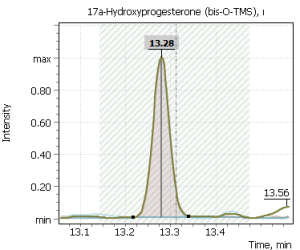
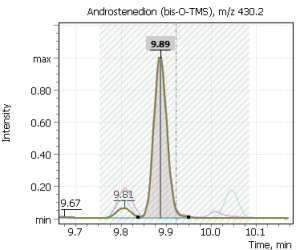
Fig. 2. Signals of target ions of steroid hormones used for building calibration dependencies.
The overall measurement procedure for steroids in biological samples
Steroid hormones are molecules (MW ≈ 300-350 Da) of a wide polarity range, resulting in their tedious and time-consuming extraction.
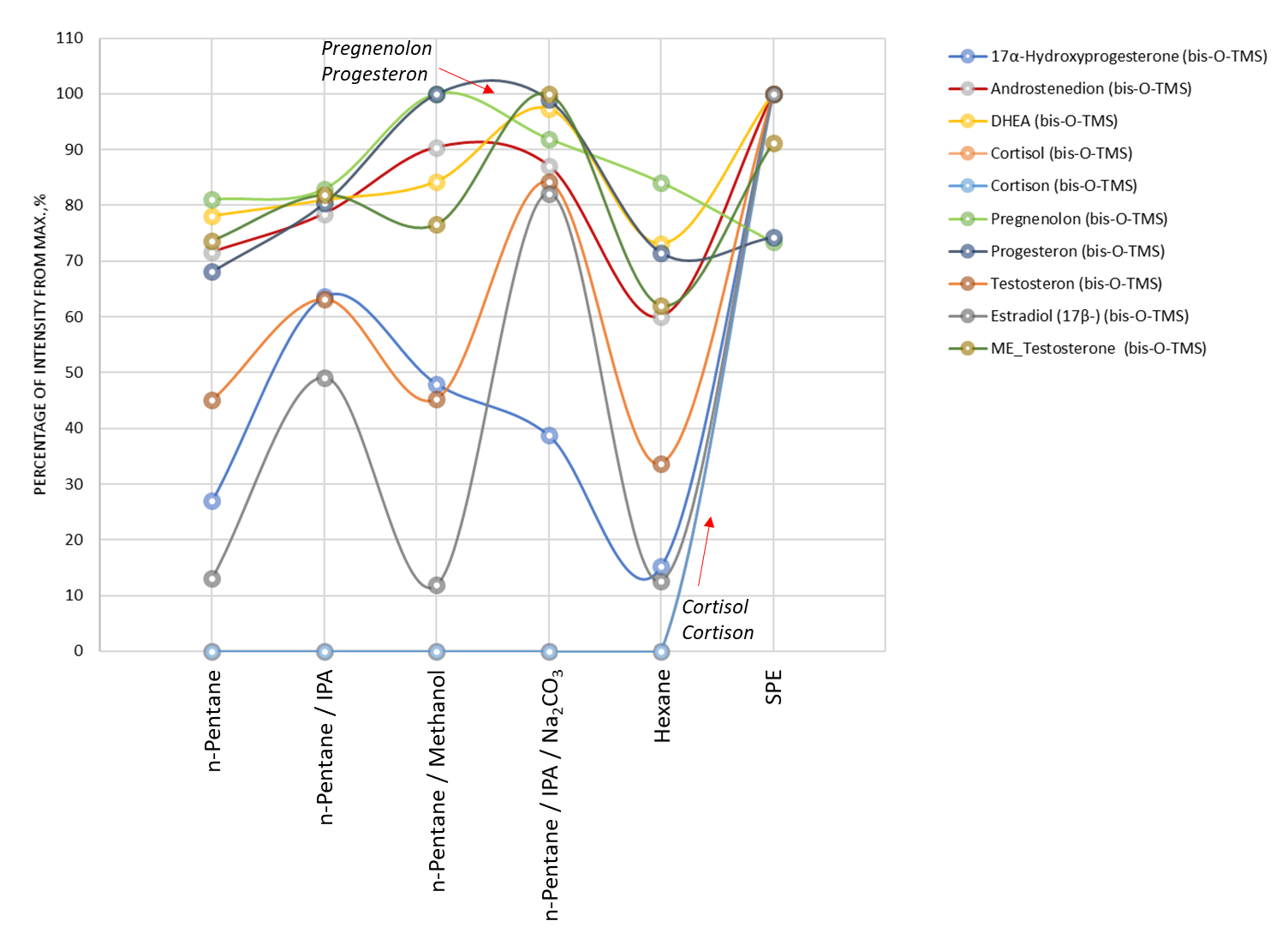
To optimize procedure for complex extraction of free steroids from saliva, several approaches of liquid-liquid extraction using n-pentane as an organic sorbent, liquid-liquid extraction under conditions of alkalinization of the mixture with sodium carbonate, and other combinations of organic solvents were tested. We also tested solid-phase extraction with C18 sorbent (AccuBond SPE ODS-C18, Agilent Technologies, Cat# 188-1310). Figure 3 shows a dotted diagram comparing different approaches to extracting steroids from a saliva sample. Most steroids show the maximum recovery with solid-phase extraction. At the same time, it is worth noting the fact that pregnenolone and progesterone were better extracted by liquid-liquid extraction in a mixture of n-pentane/methanol. Also note cortisol and cortisone were found to be successfully extracted by solid-phase extraction only, otherwise, both steroids remained in the water phase due to their high polarity.
Based on the results of the study, the procedure for solid-phase extraction of free steroids from saliva using a reversed phase based on silica gel with an octadecyl group (ODS-C18) was adopted for further work and the following elution scheme was proposed:
- The sorbent conditioning stage. Chemical activation of the solid sorbent was performed with a solution of 30% acetonitrile and 70% water. The eluate is discarded.
- Sample loading. 0 ml of the sample was applied to the sorbent. The eluate is discarded.
- Elution of target compounds. 0 ml of methanol was used as an eluting liquid. Eluate was collected in a bottle.
Fig. 3. Comparison chart of different approaches to extraction of steroids from saliva sample.
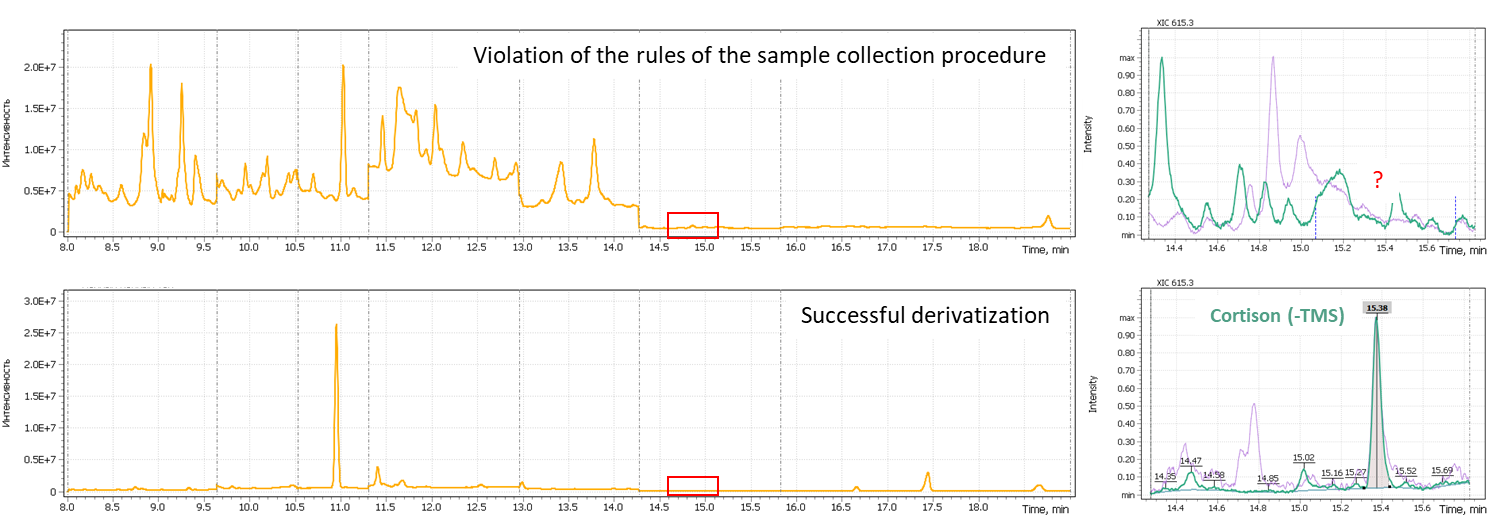
It is very important to follow specific rules of saliva sample collection as certain deviations of that will strongly impact the quality of derivatization of the dry residue of steroids later in the process. The MSTFA silylating agent is very sensitive to water, so the dried saliva extract residue should be as dehydrated as possible. The presence of food residues or protein fractions in the saliva tend to hold water strongly, no matter if a vacuum manifold used. Figure 4 shows a chromatogram of a saliva sample in case the sample collection procedure has not been properly followed and as an opposite a chromatogram with a successful sample derivatization procedure. As can be seen from the figure, when the procedure has not been followed, the chemical noise increases significantly, while target compounds signals decrease, which is a result of a low derivatization quality of target analytes. Thus, correct quantification of free steroids in such a sample turns impossible, having both the sample and the efforts had been wasted.
The use of a vacuum concentrator – Eppendorf concentrator 5301 – may become a reliable solution to eliminate cases of low derivatization efficiency. The operation of this device is based on evaporation of the solvent from the sample at an elevated temperature (60°C) and reduced pressure without freezing the initial solution. Thanks to this, the evaporation process is fast and the samples are not exposed to elevated temperatures, which is especially important when working with thermolabile biological materials.
Fig. 4. Total Ion Chromatogram of a sample with a low derivatization quality (upper figure) and that of a properly sampled saliva sample followed by acceptable quality derivatization step.
Derivatization by silylating agents implies silylation by various functional groups of the molecule. Steroid compounds, as shown in table 1, contain more than one derived functional group. In particular, estradiol contains a hydroxyl group of the aromatic ring and one hydroxyl group of the aliphatic ring. Progesterone contains two carbonyl groups, and testosterone contains a carbonyl group and an aliphatic ring hydroxyl group.
Different hormones structures imply the possibility of obtaining different derivatives, exhibiting different physical and chemical properties, that show up on the chromatogram by groups of peaks related to the same original substance.
Fig. 5. Derivatization mechanism by a silylating agent (example MSTFA, n-methyl-n (trimethylsilyl)trifluoroacetamide).
During the development of a method for rapid derivatization of steroids with several functional groups, an important task was to select concentrations and ratios of catalyzing agents, their optimal reaction temperature and time for the most complete degree of their derivatization. To tackle this task, it was proposed to use the results of the work of Jon-Aa Oh et al. [12], that proved it was possible to obtain a rapid and complete derivatization reaction using a mixture of MSTFA/NH4I (100:2) containing 1.5% dithioerythritiol for 10 minutes at 90°C. This mixture allowed the complete conversion of free steroids to TMS-substituted derivatives for the maximum possible number of functional groups. The efficiency of the derivatization method was also confirmed during this work, which is shown in the figures below.
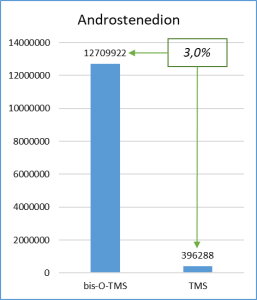
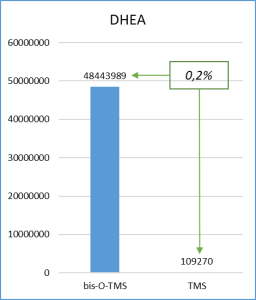
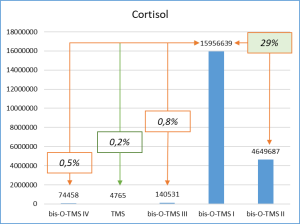
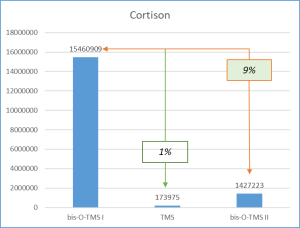
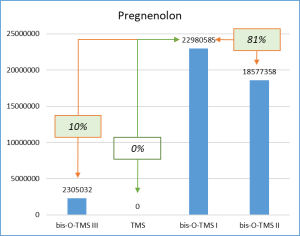
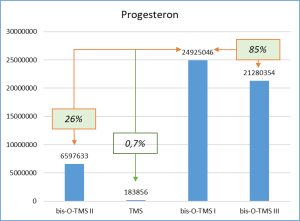
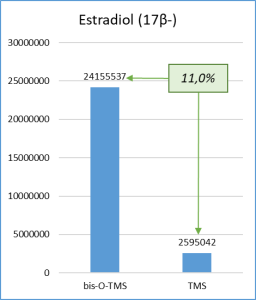
Figure 6 demonstrates the chosen derivatization method to work well for the most number of functional groups in the target molecules. Thus, the set of respective ions to further monitor was defined. In particular, only derivatives at 20% or higher level from the most intensive derivative were selected when working with target compounds.
   |
|||
 |
 |
||
 |
 |
||
 |
 |
||
Fig. 6. Percentage distribution of signals from steroid mono-, di(tri-) derivatives obtained by derivatization with a mixture of MSTFA/NH4I (100:2) containing 1.5% dithioerythritiol.
Discussion of the analysis procedure
Figure 7 shows Extracted Ions Chromatograms of target steroid compounds in human saliva sample.
Figure 6 demonstrates the chosen derivatization method to work well for the most number of functional groups in the target molecules. Thus, the set of respective ions to further monitor was defined. In particular, only derivatives at 20% or higher level from the most intensive derivative were selected when working with target compounds.
Discussion of the analysis procedure
Figure 7 shows Extracted Ions Chromatograms of target steroid compounds in human saliva sample.
Fig. 7. Mass chromatograms of target steroid hormones in saliva samples undergone the above sample preparation procedure.
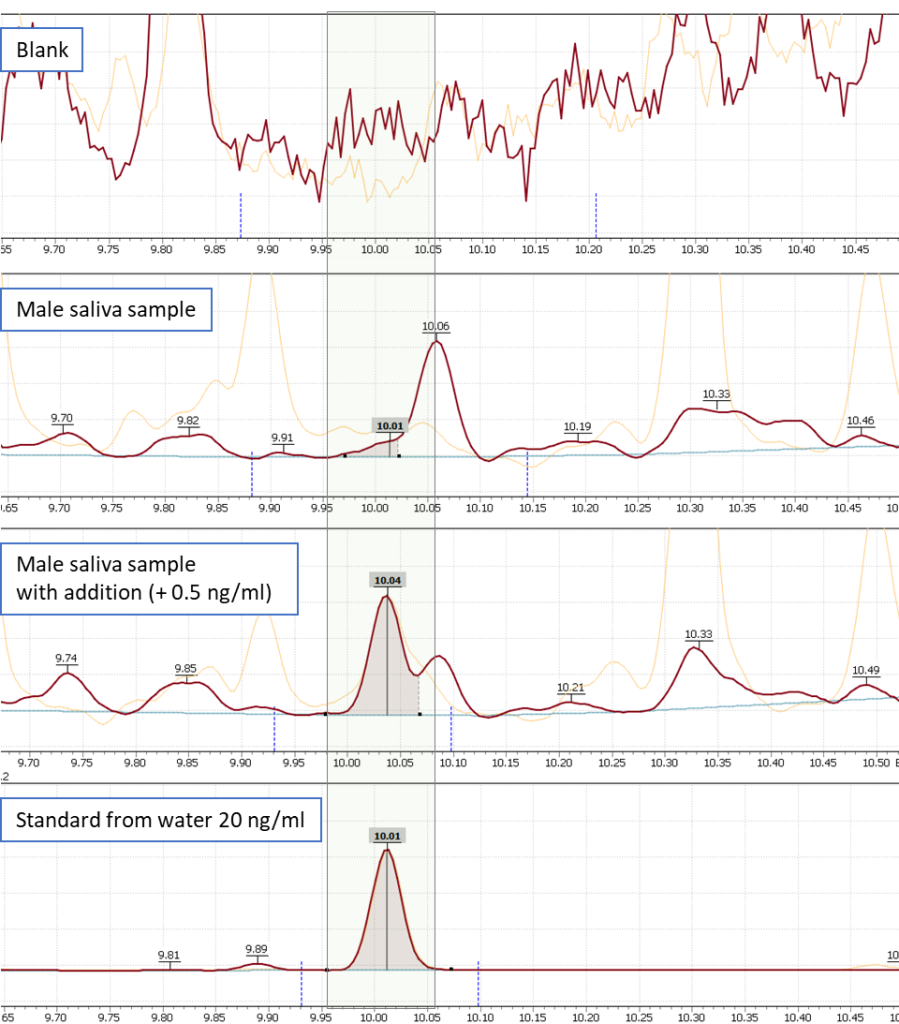
Figure 8 shows mass chromatograms of an estradiol derivative in a standard solution, a blank (model water sample), and a saliva sample of a male. Additionally, a mass chromatogram of a saliva sample with estradiol spike is presented.
Fig. 8. Comparison of estradiol ion signals in a blank sample, in a saliva sample, in a saliva sample spiked with estradiol and in a water dissolved standard.
The limits of qualitative (LOD) and quantitative (LOQ) determination of steroids with Q-Tek GC-MS single quadrupole GC-MS were calculated using a model matrix of water with known concentration levels of target compounds. Calculations were performed in accordance with the European ACAC Directive [13]. The data obtained is presented in table 4.
Table. 4. List of steroid derivatives, their linearity, and limits of qualitative (LOD) and quantitative (LOQ) detection.
| # | List of steroids derivatives | R2, linearity | LOD,
ng/mL |
LOQ,
ng/mL |
| 1 | 17α-Hydroxyprogesteron (bis-O-TMS) | 0.997 | 3.4 | 10.2 |
| 2 | 17α-Hydroxyprogesteron (mono-TMS) | 0.999 | 2.9 | 8.9 |
| 3 | Androstenedion (bis-O-TMS) | 0.999 | 3.2 | 9.7 |
| 4 | DHEA (bis-O-TMS) | 0.999 | 3.6 | 10.8 |
| 5 | Cortisol I (tetrakis-O-TMS) | 0.998 | 2.8 | 8.5 |
| 6 | Cortisol II (tetrakis -O-TMS) | 0.998 | 2.7 | 8.2 |
| 7 | Cortison (bis-O-TMS) | 0.997 | 2.9 | 8.6 |
| 8 | Pregnenolon I (tris-O-TMS) | 0.995 | 3.9 | 11.8 |
| 9 | Pregnenolon II (tris-O-TMS) | 0.996 | 3.4 | 10.2 |
| 10 | Progesteron I (tris-O-TMS) | 0.994 | 4.0 | 12.1 |
| 11 | Progesteron II (tris-O-TMS) | 0.994 | 4.0 | 12.1 |
| 12 | Progesteron III (tris-O-TMS) | 0.994 | 4.5 | 13.4 |
| 13 | Testosteron (bis-O-TMS) | 0.999 | 2.1 | 6.3 |
| 14 | Estradiol (17β-) (tris-O-TMS) | 0.999 | 2.5 | 7.6 |
Later, several tests of saliva samples were performed in this work. Clinical samples were collected from both male and female, to observe the correlation between hormones corresponding to women (estrogens (estradiol, etc.), Progestogens (pregnenolone, progesterone, etc.)) and men (androgens (testosterone, etc.)).
Figure 9 shows a comparison of the signal of the most active estrogenic (female) sex hormone – estradiol (17-beta-estradiol). It is clearly shown that the estradiol signal in the female saliva sample is significantly higher than in the male sample.
Fig. 9. Comparison of estradiol level in male and female saliva samples.
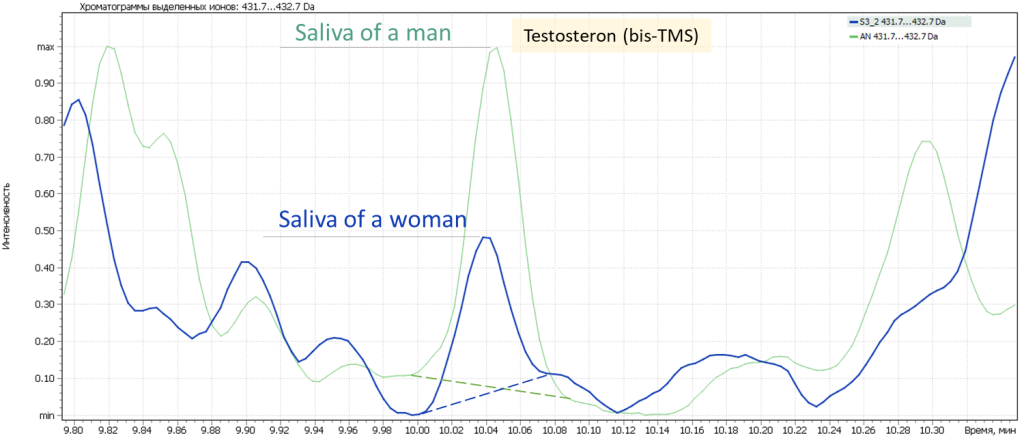
The following figure shows the same for testosterone to compare its level in the male vs female saliva. It is obvious that the testosterone signal from a male sample is almost 2-fold as high as that from a female sample, as shown in the fig. 10 below.
Fig. 10 Comparison of testosterone level in male and female saliva samples.
Conclusion
There are a number of factors that limit the widespread use of saliva in clinical labs. The new research methods application is hindered by lack of reference values of hormones level in saliva linked to the type of stimulation of saliva, gender, age, time of day and the analytical system used. [[5] This article is an example of successful application of inexpensive single quadrupole GC-MS system for steroid hormone research.
As a result of this work, a multi-component, sensitive method for analyzing steroid hormones in human saliva was developed. Optimized derivatization step resulted in minimal number of silylated derivative variants of steroids having multiple functional groups yet all variants showing good chromatographic properties. Solid-phase extraction of compounds from saliva resulted in high degree of component extraction.
It is worth noting that currently mass spectrometric methods are becoming the “gold standard” for accurate profiling of steroids, and their widespread implementation may require revision of earlier published data at some point of time. Based on literature analysis in the course of this study, a lot of efforts is still required to minimize errors at saliva sample collection and storage steps, as well as to exclude taking medications immediately prior to collecting the sample, since this can make it difficult to reliably assess the steroid profile. Thus, first of all, a single Protocol for collecting saliva, testing deadlines and analytical studies needs to be created and validated, as well as uniform reporting and availability of information about the results obtained, which is also widely discussed in the literature [5].
Analytical method developed in this work on Q-Tek GC-MS single quadrupole GC-MS system can be implemented for daily routine monitoring of the steroid profile of human saliva for large scale research on correlations with possible human diseases.
Literature
- Arregger AL, Contreras LN, Tumilasci OR, Aquilano DR, Cardoso EML: Salivary testosterone: a reliable approach to the diagnosis of male hypogonadism. Clin Endocrinol 2007, 67:656-662.
- Cardoso E, Persi G, Gonzalez N: Assessment of adrenal function by measurement of salivary steroids in response to corticotrophin in patients infected with human immunodeficiency virus. Steroids 2007, 72:328-334.
- Yasuda M, Honma S, Furuya K: Diagnostic significance of salivary testosterone measurement revisited: using liquid chromatography/mass spectrometry and enzyme-linked immunosorbent assay. J Men Heal 2008, 5:56-63.
- Shibayama Y, Higashi T, Shimada K: Simultaneous determination of salivary testosterone and dehydroepiandrosterone using LC-MS/MS: Method development and evaluation of applicability for diagnosis and medication for late-onset hypogonadism. J Chromatogr B 2009, 877:2615-2623.
- Вавилова, Т.П. Возможности и перспективы исследования гормонов в слюне / Т.П. Вавилова, И.Г. Островская, А.Е. Медведев. – Биомедицинская химия, 2014 том 60, вып. 3, с. 295-307.
- Рыбакова М.Г. Функциональная морфология больших слюнных желез в норме и при патологии эндокринной системы: Дисс. д.м.н.- Л., 1984.- 529с
- Афанасьев В.В. Сиаладенит (этиология, патогенез, клиника, диагностика и лечение). Эксперим.-клинич. исслед.: Дисс. д.м.н. – Москва, 1993. – 372с
- Dechaume M. Endocrinologie des glandes salivaires // Presse med.- 1958 -Vol 66, № 26.- P.584-586
- Godlowski Z.Z., Сalandra J.C. Salivary glands as endocrine organs. // J Appl Physiol. 1960 Jan; 15:101-5.
- Гончаров Н.П., Кация Г.В., Добрачева А.Д., Нижник А.Н., Колесникова Г.С., Хербст В., Вестерманн Ю. Свободный тестостерон в слюне как диагностический маркер андрогенного статуса мужчин. // Андрология и генитальная хирургия. – 2006 №3.-С. 33-38.
- Дедов И.И., Гончаров Н.П., Кация Г.В., Малышева Н.М. Современные биотехнологии определения тестостерона для оценки репродуктивной функции мужчин. –М: Издательство «АдамантЪ», 2010. -36 с.
- Jin-Aa Oh, Ho-Sang Shin: Rapid determination of natural steroidal hormones in saliva for the clinical diagnoses. Chemistry Central Journal 2012, 6:22
Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food, 2016